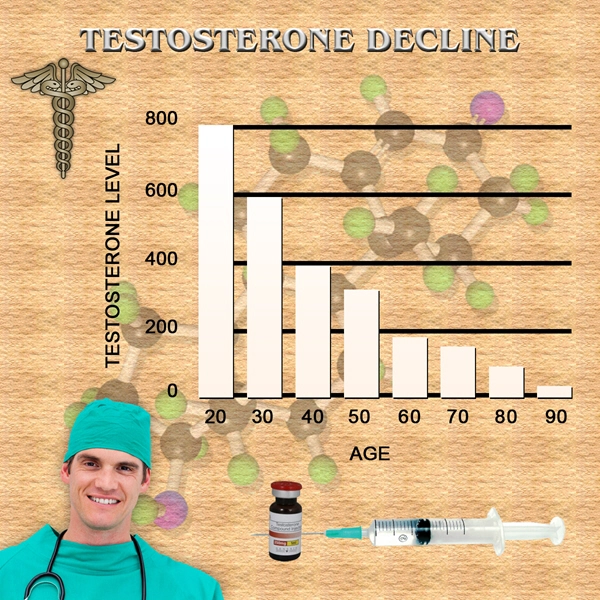
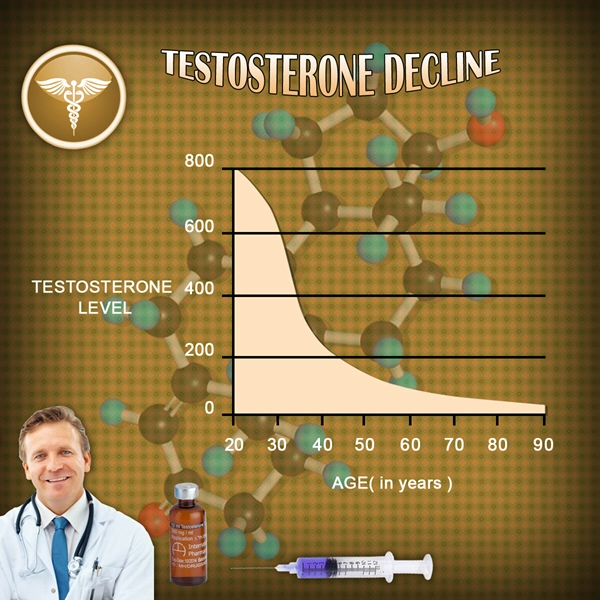
St. Louis, Missouri (PRWEB) November 25, 2014
As a personal injury law firm with a focus on defective drug law, Carey Danis & Lowe have remained abreast of the news circulating on the topic of testosterone gel and testosterone-replacement therapy as they relate to patient health and safety. Testosterone gels have been placed under increased scrutiny over the past year, in large part due to a study published in the Journal of the American Medical Association (JAMA) in November 2013 whose findings showed an increase in the risk of heart attack and stroke in study participants who used testosterone.
Carey Danis & Lowe contends that, perhaps in response to the JAMA study, the Food and Drug Administration (FDA) recently made steps to address testosterone gel safety through appointing an advisory panel. This special panel of experts released their recommendations to the FDA in September 2014.
According to an article published by the Wall Street Journal in late October 2014, the panel recommended that the FDA complete two actions, the first to order drug makers to conduct studies on their particular testosterone products as they relate to cardiovascular health, and the second, to update testosterone product labeling to reflect the reality that extensive research does not exist validating the safety of testosterone products to treat low testosterone.*
The recommendations of the FDA panel introduce the right course of action, say Carey Danis & Lowe, defective drug lawyers. It is important to test testosterone gel and other testosterone products to better understand their impact on heart health. Furthermore, updating the product labeling is vital in providing healthcare providers and patients with the most up-to-date information on the safety of testosterone products, so that they can make informed health decisions.
Legal Assistance for Alleged Defective Drug Injuries
In addition to staying current on the FDA's handling of testosterone gel, the legal team and medical professionals at Carey Danis & Lowe are also following the testosterone gel litigation taking place in a federal court in Illinois (U.S. District Court for the Northern District of Illinois, In re: Testosterone Replacement Therapy Product Liability Litigation, MDL no. 2545). As the St. Louis law firm provides legal assistance and representation to individuals who claim to have been injured by testosterone gel, Carey Danis & Lowe delivers their expert analysis on timely topics for the benefit of anyone interested in defective drug law and news.
Carey Danis & Lowe is available to review legal options, compensation eligibility, and health concerns with those who claim injury from testosterone gel products.
Carey Danis & Lowe can be reached by phone at 800.721.2519.
About Carey Danis & Lowe
See the original post here:
Carey Danis & Lowe Weighs in on Testosterone Gel Safety
Contact Us Today For A Free Consultation

- Part 2: Effect of Testosterone Replacement Therapy on Prostate Tissue in Men with Late-Onset Hypogonadism (Dramatic Health) [Last Updated On: March 24th, 2018] [Originally Added On: May 7th, 2011]
- Full: Effect of Testosterone Replacement Therapy on Prostate Tissue in Men with Late-Onset Hypogonadism (Dramatic Health) [Last Updated On: May 3rd, 2023] [Originally Added On: May 7th, 2011]
- Testosterone Replacement Therapy (TRT): Optimizing Clinical Outcomes - Michael Aziz, MD [Last Updated On: November 12th, 2023] [Originally Added On: May 8th, 2011]
- Part 1: Effect of Testosterone Replacement Therapy on Prostate Tissue in Men with Late-Onset Hypogonadism (Dramatic Health) [Last Updated On: March 24th, 2018] [Originally Added On: May 8th, 2011]
- Part 4: Effect of Testosterone Replacement Therapy on Prostate Tissue in Men with Late-Onset Hypogonadism (Dramatic Health) [Last Updated On: November 12th, 2023] [Originally Added On: May 30th, 2011]
- Part 3: Effect of Testosterone Replacement Therapy on Prostate Tissue in Men with Late-Onset Hypogonadism (Dramatic Health) [Last Updated On: November 15th, 2023] [Originally Added On: June 2nd, 2011]
- Testosterone Replacement [Last Updated On: November 23rd, 2023] [Originally Added On: June 7th, 2011]
- Medical Professor on Testosterone Replacement Therapy [Last Updated On: November 17th, 2023] [Originally Added On: June 14th, 2011]
- Sean McCorkle Discusses Testosterone Replacement Therapy [Last Updated On: November 18th, 2023] [Originally Added On: July 11th, 2011]
- Use of Testosterone in Men With Prostate Cancer [Last Updated On: November 22nd, 2023] [Originally Added On: September 28th, 2011]
- Low Testosterone (Low T) - Video [Last Updated On: November 25th, 2024] [Originally Added On: December 10th, 2011]
- Transdermal Drug Delivery - Technologies, Markets, and Companies [Last Updated On: April 29th, 2025] [Originally Added On: February 2nd, 2012]
- How Testosterone Replacement Therapy Builds Muscle and Stops Pain [Last Updated On: February 4th, 2024] [Originally Added On: February 4th, 2012]
- Teva, BioSante’s Testosterone Gel for Men Wins Approval From U.S. FDA [Last Updated On: January 11th, 2018] [Originally Added On: February 15th, 2012]
- FDA approves Teva, BioSante testosterone gel [Last Updated On: January 18th, 2018] [Originally Added On: February 15th, 2012]
- Teva Fourth-Quarter Profit Rises on Cephalon Purchase [Last Updated On: January 16th, 2018] [Originally Added On: February 15th, 2012]
- Renowned Dr. Oz and the Acclaimed Financial Times Now Have Featured Dr. Lionel Bisson, Founder of ... [Last Updated On: April 27th, 2025] [Originally Added On: February 17th, 2012]
- Omaha man says testosterone replacement therapy changed his life [Last Updated On: December 15th, 2017] [Originally Added On: May 5th, 2012]
- Auxilium Pharmaceuticals, Inc. and GlaxoSmithKline LLC Enter Into a Co-Promotion Agreement for Testim® in the U.S. [Last Updated On: May 6th, 2025] [Originally Added On: May 21st, 2012]
- Hormone therapy results in weight loss [Last Updated On: January 27th, 2018] [Originally Added On: June 25th, 2012]
- Testosterone in Women-Putting Your Sex Drive Bacl On Track - Video [Last Updated On: December 31st, 2024] [Originally Added On: November 2nd, 2012]
- Men's Health PITCH: Testosterone - Video [Last Updated On: January 1st, 2025] [Originally Added On: November 2nd, 2012]
- Dr. Karron Power Appears on Nightline - Testosterone Therapy - Video [Last Updated On: January 1st, 2025] [Originally Added On: November 2nd, 2012]
- Testosterone Replacement Therapy: Who is TRT Best For? - Video [Last Updated On: January 2nd, 2025] [Originally Added On: November 2nd, 2012]
- Female sex-enhancing nasal spray undergoing clinical trials [Last Updated On: October 24th, 2015] [Originally Added On: November 2nd, 2012]
- Andropause: A Diagnosis Whose Time Has Come [Last Updated On: June 3rd, 2025] [Originally Added On: November 16th, 2012]
- Dealing With Mood Disorders During the Holidays (Depression, Anxiety, Depersonalization) - Video [Last Updated On: February 7th, 2025] [Originally Added On: November 26th, 2012]
- Testosterone Roundtable -- Hypergonadism and Testosterone Replacement Therapy (Part 6) - Video [Last Updated On: February 19th, 2025] [Originally Added On: December 10th, 2012]
- Battling my Testosterone Replacement Therapy Doctor, Carpal Tunnel Syndrome, and Weightlifting - Video [Last Updated On: March 12th, 2013] [Originally Added On: March 12th, 2013]
- Low T? Testosterone Replacement Therapy - Video [Last Updated On: March 13th, 2013] [Originally Added On: March 13th, 2013]
- Testosterone Replacement Therapy: Nothing To Be Ashamed Of: Strike First Nutrition - Video [Last Updated On: May 18th, 2013] [Originally Added On: May 18th, 2013]
- Testosterone Replacement Therapy: Symptoms of Low Testosterone - Strike First Nutrition - Video [Last Updated On: May 18th, 2013] [Originally Added On: May 18th, 2013]
- Testosterone Replacement Therapy Testimonial - Video [Last Updated On: June 15th, 2013] [Originally Added On: June 15th, 2013]
- Taurus Male Clinic Testosterone Replacement Therapy - Video [Last Updated On: July 28th, 2013] [Originally Added On: July 28th, 2013]
- Can I Quit TRT Or Testosterone Replacement Therapy? By Low Testosterone Expert Dr David Asher - Video [Last Updated On: March 16th, 2017] [Originally Added On: August 7th, 2013]
- What Are The Side Effects of TRT Testosterone Replacement Therapy? By Low T Expert Dr. David Asher - Video [Last Updated On: August 7th, 2013] [Originally Added On: August 7th, 2013]
- Bill Jones 1513 Testosterone Replacement Therapy Testimonial - Video [Last Updated On: August 8th, 2013] [Originally Added On: August 8th, 2013]
- 2013-08-06 Testosterone Replacement Therapy - Video [Last Updated On: August 10th, 2013] [Originally Added On: August 10th, 2013]
- Testosterone Replacement Therapy West Palm Beach Florida - Video [Last Updated On: August 18th, 2013] [Originally Added On: August 18th, 2013]
- Testosterone Replacement Therapy Testimony from Patient of Body Renew Medical in Lees Summit MO - Video [Last Updated On: September 2nd, 2013] [Originally Added On: September 2nd, 2013]
- FGSW - An Update On My Testosterone Replacement Therapy: Doctor's Visit 09.16.13 - Video [Last Updated On: September 19th, 2013] [Originally Added On: September 19th, 2013]
- Testosterone Replacement Therapy - Testosterone Treatment [Last Updated On: March 25th, 2025] [Originally Added On: October 31st, 2013]
- Testosterone Side Effects from Testosterone Replacement Therapy [Last Updated On: January 11th, 2018] [Originally Added On: November 3rd, 2013]
- FGSW - An Update On My Testosterone Replacement Therapy (TRT) 11.08.13: Getting No Sleep! - Video [Last Updated On: November 14th, 2013] [Originally Added On: November 14th, 2013]
- WebMD: Erectile Dysfunction: Testosterone Replacement Therapy [Last Updated On: December 6th, 2017] [Originally Added On: November 25th, 2013]
- Testosterone Replacement Therapy! Male Hormones! [Last Updated On: January 15th, 2018] [Originally Added On: November 25th, 2013]
- FGSW - Testosterone Replacement Therapy TRT Update: Am I Back Where I Started - 11.25.13 - Video [Last Updated On: November 27th, 2013] [Originally Added On: November 27th, 2013]
- The benefits and risks of testosterone replacement therapy: a ... [Last Updated On: January 12th, 2018] [Originally Added On: December 8th, 2013]
- Testosterone replacement therapy can carry health risks - CBS News [Last Updated On: January 16th, 2018] [Originally Added On: December 8th, 2013]
- Reclaim Your Energy and Sex Drive | Testosterone Replacement ... [Last Updated On: April 6th, 2025] [Originally Added On: December 21st, 2013]
- Low Testosterone Therapy and Treatment - Do You Have Low ... [Last Updated On: October 24th, 2015] [Originally Added On: December 24th, 2013]
- How Long Does it Take for Testosterone Replacement Therapy to ... [Last Updated On: December 30th, 2017] [Originally Added On: January 3rd, 2014]
- Testosterone Therapy - Bioidentical Testosterone Replacement [Last Updated On: January 23rd, 2018] [Originally Added On: January 9th, 2014]
- Transdermal testosterone replacement therapy - Video abstract 43475 - Video [Last Updated On: January 11th, 2014] [Originally Added On: January 11th, 2014]
- Testosterone Therapy - Bioidentical Testosterone Replacement ... [Last Updated On: December 21st, 2017] [Originally Added On: January 13th, 2014]
- Testosterone Side Effects from Testosterone Replacement ... [Last Updated On: December 8th, 2017] [Originally Added On: January 20th, 2014]
- MyAntiAgingMD The Leader In Testosterone Replacement Therapy - Video [Last Updated On: October 21st, 2020] [Originally Added On: January 29th, 2014]
- The benefits and risks of testosterone replacement therapy ... [Last Updated On: October 15th, 2020] [Originally Added On: February 6th, 2014]
- The Secret Female Hormone: How Testosterone Replacement Can Change Your Life [Last Updated On: October 28th, 2020] [Originally Added On: February 11th, 2014]
- Testosterone Replacement Therapy found to be linked to Heart Problems [Last Updated On: April 19th, 2025] [Originally Added On: February 25th, 2014]
- Serious Side Effects Linked to Testosterone Therapy [Last Updated On: September 17th, 2025] [Originally Added On: February 27th, 2014]
- Doctor Reveals How Getting off Testosterone Will Hurt Fighters on It [Last Updated On: November 27th, 2020] [Originally Added On: March 4th, 2014]
- California joins Nevada in banning testosterone replacement therapy [Last Updated On: October 4th, 2020] [Originally Added On: March 6th, 2014]
- Testosterone Replacement Therapy: How to Administer Expert TRT By John K. Crisler, DO - Video [Last Updated On: October 31st, 2020] [Originally Added On: March 9th, 2014]
- FGSW - Week 14 Weigh-In & Update: Going Back To The Endocrinologist - Video [Last Updated On: March 29th, 2014] [Originally Added On: March 29th, 2014]
- Drug Recall Attorney at Herrera Law Firm, Inc., Comments on Reported Link Between Testosterone Drugs and Heart Attack ... [Last Updated On: November 30th, 2020] [Originally Added On: April 2nd, 2014]
- FGSW - Testosterone Replacement Therapy Update 03.27.14: An Improvement?? - Video [Last Updated On: October 8th, 2020] [Originally Added On: April 2nd, 2014]
- FGSW Weekly Weigh In & Update 15 Still Working On Mobility 03 31 14 - Video [Last Updated On: October 15th, 2020] [Originally Added On: April 5th, 2014]
- Discover the risk of prostate cancer with testosterone replacement therapy - Video [Last Updated On: October 1st, 2020] [Originally Added On: April 5th, 2014]
- FGSW - Weekly Weigh In & Update 15: Still Working On Mobility 03.31.14 - Video [Last Updated On: October 3rd, 2020] [Originally Added On: April 7th, 2014]
- Testosterone Replacement Therapy - Video [Last Updated On: October 1st, 2020] [Originally Added On: April 12th, 2014]
- Reclaim Your Energy and Sex Drive | Testosterone ... [Last Updated On: April 21st, 2025] [Originally Added On: April 14th, 2014]
- Low Testosterone Claims: Options in a Growing Class Action Suit - Video [Last Updated On: November 25th, 2020] [Originally Added On: April 26th, 2014]
- Acrux falls 15% on sales warning [Last Updated On: September 28th, 2025] [Originally Added On: April 28th, 2014]
- Testosterone Replacement Therapy in Men - myVMC [Last Updated On: November 1st, 2020] [Originally Added On: April 30th, 2014]
- NJ-Based Law Firm Exploring Potential Legal Claims from Side Effects of Testosterone Replacement Therapy [Last Updated On: October 22nd, 2020] [Originally Added On: May 1st, 2014]
- Viewer Mail - Testosterone Replacement Therapy/TRT Not Natty, Balls Busted, etc. - Video [Last Updated On: October 2nd, 2020] [Originally Added On: May 4th, 2014]
- Seen At 11: Experts Urge Caution When Using Popular Hormone Replacement Therapy [Last Updated On: November 11th, 2020] [Originally Added On: May 10th, 2014]
- Are Low-T Drugs Putting Patients At High Risk? [Last Updated On: October 15th, 2020] [Originally Added On: May 10th, 2014]
- Seen At 11: Testosterone Replacement Therapy Could Come With Serious Side Effects - Video [Last Updated On: May 11th, 2014] [Originally Added On: May 11th, 2014]
Word Count: 446