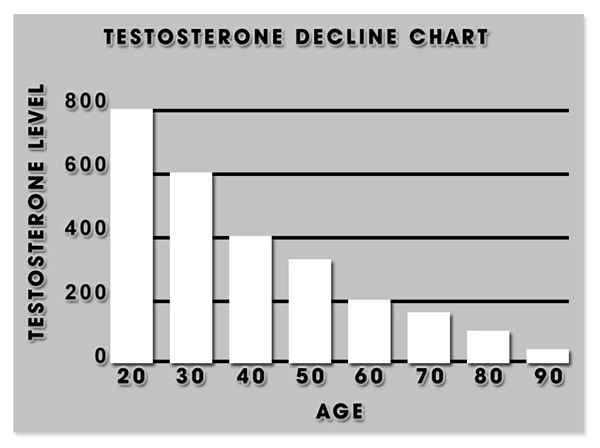
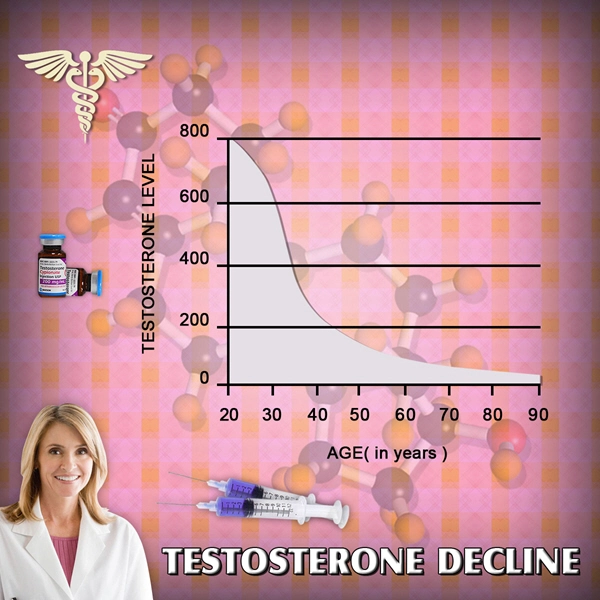
DeLand, FL (PRWEB) January 22, 2014
*To see if you qualify for this Hepatitis C Clinical Trial in Florida, visit Avail Clinical Research on the web (http://www.availclinical.com) or contact us directly at (386) 785-2404. There is no cost to participate, no insurance is required, and you may receive compensation for time and travel.
STUDY DESIGN
The study will be conducted in three parts. Part A will utilize a randomized group design in treatment-naive, GT!b and 4 HCV-infected subjects. Sixty subjects will be enrolled and randomized to groups of equal size. GT!b and GT4 HCV-infected subjects will be stratified by genotype across the treatment arms. Each group will receive the study drugs for up to 12 weeks followed by a 24-week follow-up to determine sustained virologic response (SVR).
Part B will be a randomized, open-label, parallel-group design in treatment-naive, GT!b, 4 and 6 HCV-infected subjects. Enrollment into Part B will commence following enrollment completion of Part A. GT!b and GT4 HCV-infected subjects will be stratified by genotype between treatment arms in Cohorts I b and 2b. Per Amendment 5, enrollment into the I00 mg RBV-free arm was capped at the number of subjects who had already been dosed into that cohort, and RBV was immediately added to their treatment regimen.
Part C will be an open-label, randomized, parallel group design in treatment-naive or IFN/RBV-treatment relapsed, GT!a and GT!b HCV-infected subjects. An independent DSMB will review the available PK, safety, and antiviral activity data after all subjects have completed 4 weeks of treatment.
BACKGROUND & RATIONALE
Hepatitis C virus (HCV) infection is a global public health problem. The global prevalence of chronic hepatitis C infection is estimated to be approximately 150 million HCV-infected persons worldwide. An estimated 60-70% of chronically infected people develop chronic liver disease; 5-20% develop cirrhosis and 1-5% die from cirrhosis or liver cancer. In 25% of liver cancer patients, the underlying cause is hepatitis C.
This new hepatitis C drug is being developed as a novel, HCV nonstructural protein S A (NSSA) inhibitor agent for the therapy of chronic hepatitis C. This drug acts as a potent inhibitor of HCV replication, inhibiting HCV of Genotypes (GT) l a, l b, 2a, 3a, 4a and Sa in vitro with half maximal effective concentration (ECso) values ranging from 2 to 24 pM, suggesting that it has pan-genotypic activity.
PRIMARY OBJECTIVES
Contact Us Today For A Free Consultation

- Cano's spokeswoman was client of Biogenesis [Last Updated On: January 25th, 2024] [Originally Added On: May 4th, 2013]
- Documents: Cano associate was client of clinic [Last Updated On: January 25th, 2024] [Originally Added On: May 4th, 2013]
- Sources: Cano associate was Biogenesis client [Last Updated On: January 25th, 2024] [Originally Added On: May 4th, 2013]
- Early Stage Testicular Cancer - Surveillance Is Best Follow-Up Strategy [Last Updated On: January 25th, 2024] [Originally Added On: May 18th, 2013]
- 2013 Endocrine Function Testing Market in Europe: Hospitals, Commercial Labs, Physician Offices, Ambulatory Care Centers [Last Updated On: January 25th, 2024] [Originally Added On: June 14th, 2013]
- Europe Endocrine Function Testing Market Studied by VPG in Cutting-Edge Report Now Available at MarketPublishers.com [Last Updated On: January 25th, 2024] [Originally Added On: June 18th, 2013]
- A More 'Natural' Version Of IVF Proves A Success [Last Updated On: January 25th, 2024] [Originally Added On: June 19th, 2013]
- Weight Loss Cure with Metabolic Cookbook - Video [Last Updated On: January 25th, 2024] [Originally Added On: July 12th, 2013]
- Weight Loss Drops - Are They A Scam? - Video [Last Updated On: January 25th, 2024] [Originally Added On: July 12th, 2013]
- Abbott Features Solutions to Help Labs Prepare for the Evolving Healthcare Landscape at the American Association for ... [Last Updated On: January 25th, 2024] [Originally Added On: July 30th, 2013]
- How Testicular Cancer Is Diagnosed | Testicular Cancer - Video [Last Updated On: January 25th, 2024] [Originally Added On: August 11th, 2013]
- 2014 Opportunities in the US Clinical Chemistry and Immunodiagnostics Markets [Last Updated On: January 25th, 2024] [Originally Added On: September 27th, 2013]
- Serie A - Doping ban overturned on cancer sufferer Acerbi [Last Updated On: January 25th, 2024] [Originally Added On: January 8th, 2014]
- Health Highlights: Jan. 8, 2014 [Last Updated On: January 25th, 2024] [Originally Added On: January 8th, 2014]
- Testosterone Replacement Therapy [Last Updated On: December 9th, 2023] [Originally Added On: January 19th, 2014]
- Luteal Phase: The Uterine Lining Phase - Video [Last Updated On: January 25th, 2024] [Originally Added On: April 10th, 2014]
- Doping case against Acerbi dismissed [Last Updated On: January 25th, 2024] [Originally Added On: April 15th, 2014]
- Drugs that Cause Gynecomastia [Last Updated On: January 25th, 2024] [Originally Added On: May 14th, 2014]
- Illegal Online Meds Targeted in Worldwide Crackdown, FDA Says [Last Updated On: January 25th, 2024] [Originally Added On: May 24th, 2014]
- Biogenesis' Bosch surrenders in PEDs case [Last Updated On: January 25th, 2024] [Originally Added On: August 5th, 2014]
- Duchess of Cambridge 'hugely disappointed' after being forced to pull out of yet another engagement due to morning ... [Last Updated On: January 25th, 2024] [Originally Added On: October 1st, 2014]
- Kate, Duchess of Cambridge dazzles in baby blue gown at the Natural History Museum [Last Updated On: January 25th, 2024] [Originally Added On: October 22nd, 2014]
- 'They're poisoning us'. How religious leaders are hindering vaccination programmes across the world [Last Updated On: January 25th, 2024] [Originally Added On: November 14th, 2014]
- Poor prognosis germ-cell tumours are only cured in about half of patients. We aimed to assess whether treatment ... [Last Updated On: January 25th, 2024] [Originally Added On: November 23rd, 2014]
- Nursing a Grudge [Last Updated On: January 25th, 2024] [Originally Added On: January 22nd, 2015]
- Hormone Levels in Men - Testosterone Injections [Last Updated On: November 30th, 2021] [Originally Added On: September 9th, 2016]
- Human Growth Hormone May Actually IMPROVE Quadricep Strength After Reconstruction of Torn ACL [Last Updated On: September 23rd, 2024] [Originally Added On: June 22nd, 2020]
- Six Ways to Feel Good and Balance Your Hormones at the Same Time! [Last Updated On: January 25th, 2024] [Originally Added On: September 24th, 2020]
- Your Birthplace Heavily Influences Your Future Testosterone Levels [Last Updated On: August 19th, 2024] [Originally Added On: March 6th, 2021]
- Both High and Low Levels of Testosterone Correlate With Cardiovascular Issues in Men [Last Updated On: September 27th, 2024] [Originally Added On: April 14th, 2021]
- Breaking News: Testosterone May Be the Answer to Autoimmune Diseases [Last Updated On: August 24th, 2024] [Originally Added On: May 14th, 2021]
- Non-Stop Cravings for Protein? Blame it on Your Gut Hormones! [Last Updated On: September 13th, 2024] [Originally Added On: May 21st, 2021]
- Testosterone Therapy Could Help Quell Your Asthma Attacks [Last Updated On: September 12th, 2024] [Originally Added On: June 20th, 2021]
- BPA Here, BPA There, What’s the Reason for the Scare? [Last Updated On: September 16th, 2024] [Originally Added On: June 29th, 2021]
- Low Testosterone Linked to Depression and Suicidal Ideation [Last Updated On: August 25th, 2024] [Originally Added On: July 5th, 2021]
- It’s True: Men Today Have Less Testosterone Compared to Men a Generation Ago [Last Updated On: September 19th, 2024] [Originally Added On: July 13th, 2021]
- Losing Weight with Baratric Surgery Reverses Low Testosterone [Last Updated On: October 2nd, 2024] [Originally Added On: February 8th, 2022]
- Do Larger Testicles Make More Testosterone? [Last Updated On: July 24th, 2024] [Originally Added On: May 3rd, 2022]
- Fact or Myth: Have Testosterone Levels Really Dropped by 50% Just in the Past Two Decades? [Last Updated On: August 14th, 2024] [Originally Added On: June 4th, 2022]
- Another Analysis Shows No Cardiovascular Risks With Testosterone Therapy [Last Updated On: October 6th, 2024] [Originally Added On: November 27th, 2022]
Word Count: 406