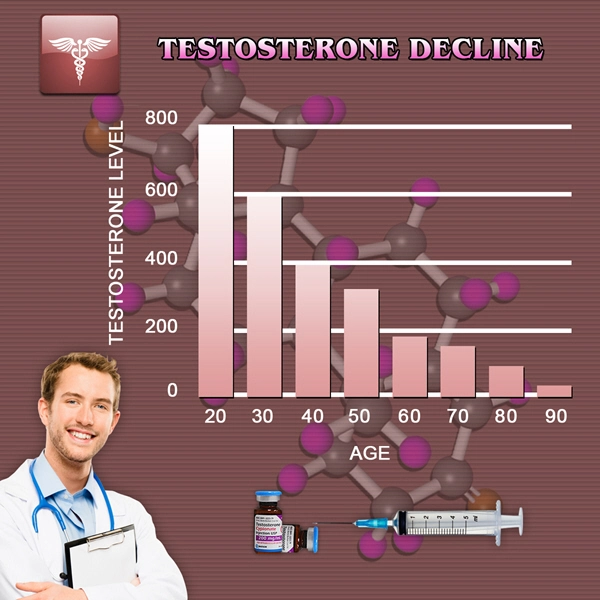
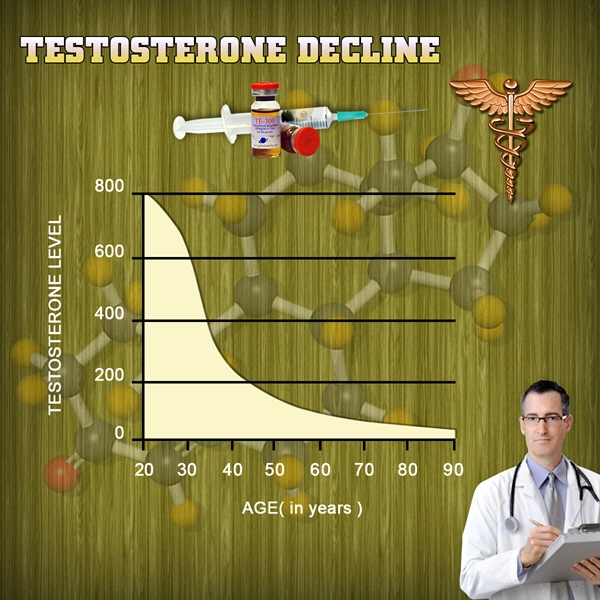
CARLSBAD, Calif., July 15, 2013 /PRNewswire/ -- Qualigen, Inc., a privately owned company that provides rapid, easy to use, fully quantitative diagnostic testing solutions, announced today that it has received 510(k) clearance from the United States Food and Drug Administration (FDA) for its FastPack Vitamin D Immunoassay for use on its proprietary FastPack System for the rapid determination of vitamin D status. FastPack Vitamin D test results are available in 10 minutes, providing a significant benefit to clinicians and patients. Numerous studies have shown a high global prevalence of vitamin D insufficiency and the measurement of vitamin D status provides opportunities for preventive and therapeutic interventions. Insufficient vitamin D levels are a cause of secondary hyperparathyroidism and diseases resulting in impaired bone metabolism (like rickets, osteoporosis, osteomalacia) and have been associated with an increasing risk of many chronic diseases, including common cancers, autoimmune or infectious diseases or cardiovascular problems. In terms of market size, in 2013, the number of vitamin D tests performed worldwide is estimated to be greater than 100 million. By 2015, this number is expected to reach almost 140 million tests.
"We are confident that our FastPack Vitamin D Immunoassay will provide customers with a rapid, highly accurate and easy to use solution for the assessment of vitamin D status in their patients and will generate significantly increased utilization of the FastPack System throughout our installed base of FastPack Analyzers," said Paul Rosinack, president and chief executive officer of Qualigen, Inc. "In addition, we expect the introduction of this new test to drive an increase in new FastPack System placements worldwide."
The FastPack System is the only fully-quantitative immunoassay system which features one-touch operation and provides high quality test results in 10 minutes or less, depending upon the assay. This patented technology platform combines a novel analyzer and pouch-based disposable with powerful monoclonal antibody, magnetic particle chemiluminescence to provide test results that are highly sensitive, accurate and precise. The addition of Vitamin D expands the FastPack menu to eight assays: Prostate Specific Antigen (PSA), free PSA (FPSA), Testosterone, Thyroid Stimulating Hormone (TSH), free Thyroxine (FT4), Human Chorionic Gonadotropin (hCG), Alpha GST and Vitamin D.
The 510(k) clearance allows Qualigen to market and sell its new FastPack Vitamin D Immunoassay in the United States. In addition, the FastPack Vitamin D Immunoassay is CE Marked for sale in the EU.
About Qualigen, Inc.
Qualigen, Inc. develops, manufactures and markets the FastPack System, the first custom-designed chemiluminescent immunoassay platform to perform complex, fully-quantitative immunoassays quickly, easily and at the touch of a button. The FastPack System provides rapid diagnostic information that can improve the health and well-being of people worldwide, through better patient outcomes and economic benefits to the healthcare system. For more information about Qualigen's FastPack System, visit http://www.qualigeninc.com.
The rest is here:
Qualigen Receives FDA Clearance for Its FastPack® Vitamin D Immunoassay
Contact Us Today For A Free Consultation

- Weight Loss Cure - Metabolic Cookbook - Video [Last Updated On: January 25th, 2024] [Originally Added On: July 12th, 2013]
- How Julia Montes lost weight [Last Updated On: January 25th, 2024] [Originally Added On: October 4th, 2013]
- Home - Dr. Jenyons Medical Weight Loss and Rejuvenation Center [Last Updated On: January 25th, 2024] [Originally Added On: December 8th, 2013]
- Dieters Beware: Weight Loss Products May Use Deceptive Marketing [Last Updated On: January 25th, 2024] [Originally Added On: January 8th, 2014]
- FTC Announces $34 Million in Settlements with Companies Over Bogus Weight-Loss Products [Last Updated On: January 25th, 2024] [Originally Added On: January 9th, 2014]
- The Point of P3 : #3 Food Sensitivities Discovered to Help Weight Maintenance - Video [Last Updated On: January 25th, 2024] [Originally Added On: May 19th, 2014]
- The Weight Loss Black List; Twelve Dangerous Weight Loss Ingredients [Last Updated On: January 25th, 2024] [Originally Added On: May 31st, 2014]
- Getting a degree in dieting [Last Updated On: January 25th, 2024] [Originally Added On: June 10th, 2014]
- Eat your way to a hot body [Last Updated On: January 25th, 2024] [Originally Added On: June 21st, 2014]
- 7 Fad Diets You Shouldn't Try [Last Updated On: January 25th, 2024] [Originally Added On: July 19th, 2014]
- Try the cookie diet? [Last Updated On: January 25th, 2024] [Originally Added On: July 25th, 2014]
- 14 Fad Diets You Shouldnt Try [Last Updated On: January 25th, 2024] [Originally Added On: July 26th, 2014]
- B12 Injection Therapy as Part of Hormone Replacement Therapy - Video [Last Updated On: January 25th, 2024] [Originally Added On: August 12th, 2014]
- FTC continues cracking down on weight loss scams [Last Updated On: January 25th, 2024] [Originally Added On: December 13th, 2014]
- Micronutrients: The Gateway to Cellular Health Function 101 [Last Updated On: March 1st, 2024] [Originally Added On: June 24th, 2020]
- Could a Revolutionary Weight Loss Strategy Be on the Horizon? [Last Updated On: February 24th, 2024] [Originally Added On: August 19th, 2020]
- Controlling Testosterone Levels Through Diet [Last Updated On: February 12th, 2024] [Originally Added On: October 12th, 2020]
- Selecting the Ideal Intermittent Fasting Protocol [Last Updated On: January 25th, 2024] [Originally Added On: October 20th, 2020]
- Limiting the Influence of Estrogen on Male Hormone Balance Through Diet [Last Updated On: January 25th, 2024] [Originally Added On: December 31st, 2020]
- Vitamin D and Sunlight for Mood and Hormone Balance [Last Updated On: January 25th, 2024] [Originally Added On: February 22nd, 2021]
- Testosterone-Boosting Foods: Nutrition for a Man [Last Updated On: September 18th, 2024] [Originally Added On: March 24th, 2021]
- Boost Your Testosterone With a Low-Calorie, Ketogenic-Focused Diet [Last Updated On: August 30th, 2024] [Originally Added On: June 1st, 2021]
- The Standard American Diet is Linked to Poor Testicular Function [Last Updated On: August 23rd, 2024] [Originally Added On: June 8th, 2021]
- Drinking Water Alone Isn’t Enough [Last Updated On: August 26th, 2024] [Originally Added On: March 22nd, 2022]
- A High-Protein Diet – Good for Testosterone Levels or Not? [Last Updated On: September 3rd, 2024] [Originally Added On: March 29th, 2022]
- Inflammatory Foods Linked to Low Testosterone [Last Updated On: September 5th, 2024] [Originally Added On: July 18th, 2022]
- Vitamins and Nutrients that Keep Hair Healthy and Vibrant [Last Updated On: August 11th, 2024] [Originally Added On: November 23rd, 2022]
- Are You a “Soy Boy”? [Last Updated On: December 3rd, 2023] [Originally Added On: February 10th, 2023]
- Finding the Origin of Prostate Cancer to Stop Cancer Before it Starts [Last Updated On: October 25th, 2024] [Originally Added On: February 13th, 2023]
- How to burn calories while walking. [Last Updated On: February 20th, 2024] [Originally Added On: June 27th, 2023]
Word Count: 468