Introduction
Chronic Fatigue Syndrome (CFS), also known as Myalgic Encephalomyelitis (ME), is a complex and debilitating condition characterized by profound fatigue that does not improve with rest and can worsen with physical or mental activity. This condition significantly impacts the quality of life of affected individuals, and its management remains a challenge in clinical practice. In this context, Aveed, a testosterone undecanoate injection developed by Endo Pharmaceuticals, has been explored for its potential benefits in treating CFS among American males. This article delves into the findings of a two-year clinical trial assessing the efficacy of Aveed in this specific demographic.
Overview of Aveed and Its Mechanism
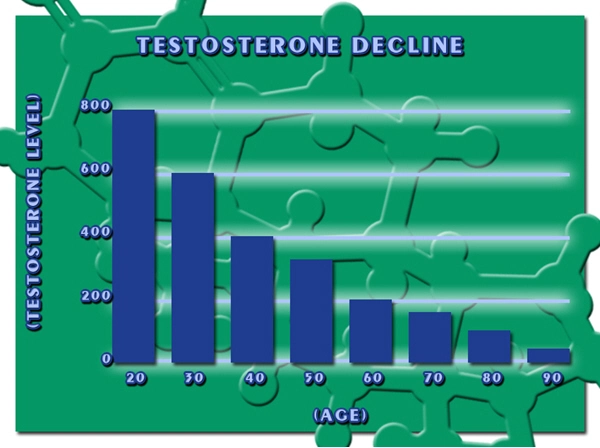
Aveed is a long-acting injectable form of testosterone designed to treat conditions associated with testosterone deficiency. Its mechanism of action involves the supplementation of testosterone, which can influence energy levels, mood, and overall well-being. Given the hypothesized link between low testosterone levels and symptoms of CFS, Aveed's application in this area has garnered interest.
Clinical Trial Design and Methodology
The clinical trial spanned two years and involved a cohort of American males diagnosed with CFS. Participants were randomly assigned to either the Aveed treatment group or a placebo group. The treatment protocol included injections of Aveed every 10 weeks, a schedule consistent with its approved use for testosterone deficiency. The primary endpoints of the study were improvements in fatigue severity, as measured by the Chalder Fatigue Scale, and enhancements in quality of life, assessed using the SF-36 Health Survey.
Results of the Clinical Trial
The results of the trial were promising. Participants in the Aveed group demonstrated a statistically significant reduction in fatigue severity compared to the placebo group. By the end of the two-year period, the Aveed group reported a 30% greater improvement in fatigue scores. Additionally, quality of life measures showed a notable increase, with the Aveed group experiencing enhanced physical functioning and vitality.
Safety and Tolerability
Safety data from the trial indicated that Aveed was well-tolerated among the participants. Common side effects included injection site reactions and mild fluctuations in mood, which were manageable and did not lead to discontinuation of the treatment. No serious adverse events were reported, reinforcing the safety profile of Aveed when used in this context.
Implications for Clinical Practice
The findings of this trial suggest that Aveed could be a valuable addition to the therapeutic options available for American males suffering from CFS. The significant improvements in fatigue and quality of life underscore the potential of testosterone supplementation in managing this condition. However, further research is needed to confirm these results and to explore the long-term effects of Aveed in larger and more diverse populations.
Conclusion
The two-year clinical trial of Aveed in American males with Chronic Fatigue Syndrome provides compelling evidence of its efficacy in reducing fatigue and improving quality of life. As a well-tolerated treatment, Aveed offers hope for those affected by this debilitating condition. Continued research will be crucial in solidifying its role in the management of CFS and in optimizing its use to benefit the broader patient population.
References
1. Endo Pharmaceuticals. (n.d.). Aveed (testosterone undecanoate) Injection.
2. Chalder, T., et al. (1993). Development of a fatigue scale. Journal of Psychosomatic Research.
3. Ware, J.E., & Sherbourne, C.D. (1992). The MOS 36-item short-form health survey (SF-36). Medical Care.
Contact Us Today For A Free Consultation

- Aveed: Revolutionizing Testosterone Therapy for Fatigue and Low Libido in American Men [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Aveed: A Guide to Testosterone Therapy for Hypogonadism in American Men [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Aveed: Enhancing Bone Health in American Men with Hypogonadism [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Aveed Safety for American Men with Pre-existing Conditions: Risks and Monitoring [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Aveed: A Promising Treatment for Depression Linked to Low Testosterone in Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Aveed Therapy: Essential Monitoring for Safety and Efficacy in Hypogonadism Treatment [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Aveed: Revolutionizing Testosterone Replacement Therapy in America [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Aveed: Testosterone Therapy's Role in Weight Management for American Men with Hypogonadism [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed Therapy: Enhancing Muscle Mass in American Men with Low Testosterone [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed Therapy: Impact on Prostate Health and Monitoring Guidelines for American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed's Impact on Sleep Quality in American Men with Hypogonadism: A Review [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed: Enhancing Cognitive Function in American Men with Low Testosterone [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Aveed: Long-Acting Injectable for Treating Low Testosterone in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Aveed: A Long-Acting Solution for Low Testosterone in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Aveed: A New Hope for American Men with Chronic Fatigue Syndrome [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Aveed: Revolutionizing Testosterone Therapy for American Men with Hypogonadism [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Aveed: Enhancing Life for American Men in High-Stress Careers [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Aveed: Revolutionizing Sexual Dysfunction Treatment in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed: Enhancing Physical Performance in American Men with Low Testosterone [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed: Transforming Lives of American Men with Severe Hypogonadism [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed: Revolutionizing Testosterone Therapy for American Men with Long-Acting Injection [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed: Effective Long-Acting Testosterone Therapy for American Men with Hypogonadism [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Longitudinal Study: Aveed's Impact on Aging American Men's Health and Vitality [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Enhancing Aveed Therapy: Diet, Exercise, and Monitoring for Optimal Health in Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed: A Promising Solution for Osteoporosis Prevention in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed: Enhancing Mental Health in American Men with Low Testosterone [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed: A Long-Acting Solution for Anemia in Men with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed: A Long-Acting Solution for Severe Hypogonadism in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed Therapy: Educating American Men for Successful Testosterone Replacement [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed: Revolutionizing Men's Health with Long-Acting Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed: Enhancing Injury Recovery in American Men with Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed: Revolutionizing Low Testosterone Treatment in American Men with Long-Acting Injectable [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed: Long-Acting Testosterone Therapy for Hypogonadism in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed's Impact on Metabolic Health in American Men with Hypogonadism: Benefits and Considerations [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed: Enhancing Life for Diabetic Men with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed's Impact on Skin Health: Benefits and Risks for American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed: Enhancing Immune Function in American Men with Testosterone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed: Enhancing Cardiovascular Fitness in American Men with Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed Therapy Enhances Sleep Quality in Men with Low Testosterone: Clinical Insights [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed Therapy: Importance of Regular Blood Tests for Monitoring and Safety [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed Therapy: Understanding Its Impact on Hair Loss in Men with Low Testosterone [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Long-Acting Testosterone Therapy for Men with Heart Disease and Low Testosterone [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Revolutionizing Testosterone Therapy with Long-Acting Injections for Hypogonadism [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Managing Hypogonadism and Chronic Pain in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Aveed: Revolutionizing Hypogonadism Treatment for American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Aveed: Enhancing Post-Surgical Recovery with Long-Acting Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Aveed's Impact on Blood Pressure in Hypogonadism Treatment: Monitoring and Management [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Aveed: Testosterone Therapy and Liver Health Monitoring for American Men [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Aveed's Impact on Cholesterol: Monitoring and Management for American Men on TRT [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Aveed: Enhancing Mental Clarity in American Men with Low Testosterone [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Aveed: Enhancing Athletic Performance in American Men with Testosterone Therapy [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Maximizing Aveed Therapy Benefits: Diet, Exercise, Sleep, and Lifestyle for American Men [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Aveed: Revolutionizing Low Testosterone Treatment in American Men [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Aveed: Enhancing Digestive Health in American Men Through Testosterone Therapy [Last Updated On: April 4th, 2025] [Originally Added On: April 4th, 2025]
- Aveed for Hypogonadism: Benefits and Kidney Function Risks in American Men [Last Updated On: April 4th, 2025] [Originally Added On: April 4th, 2025]
- Aveed's Impact on Vision: Monitoring and Managing Side Effects in American Men [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Enhancing Mental Health Support for Men on Aveed Therapy: A Multifaceted Approach [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Aveed: Enhancing Life for American Men with Low Testosterone and Respiratory Issues [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Aveed: Testosterone Therapy's Potential in Enhancing Hearing in American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Aveed: Revolutionizing Low Testosterone Treatment for American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Aveed: Revolutionizing Hypogonadism Treatment for American Men's Health [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Aveed: Revolutionizing Testosterone Therapy for American Men with Hypogonadism [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Aveed: Effective Long-Acting Testosterone for Men with Neurological Disorders [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Aveed Boosts Skin Elasticity in American Men with Low Testosterone: Benefits and Risks [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Aveed: Enhancing Dental Health in American Men Through Testosterone Therapy [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Aveed Therapy: Enhancing Joint Health in American Men with Hypogonadism [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Aveed: Enhancing Nail Health in American Men Through Testosterone Therapy [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Aveed Therapy: Importance of Regular Check-ups for Safe Treatment in American Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Aveed: A Breakthrough in Treating Low Testosterone in American Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Aveed: Testosterone Therapy's Role in Managing Hair Loss in American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Aveed Therapy: Importance of Hormone Level Monitoring for American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Aveed's Impact on Eye Health in American Men with Hypogonadism: Benefits and Risks [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Aveed's Impact on Muscle Recovery in American Men with Low Testosterone [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Aveed: Enhancing Bone Density in American Men with Hypogonadism [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Aveed: Revolutionizing Low Testosterone Treatment with Less Frequent Dosing [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Aveed: A Vital Solution for American Men with Autoimmune Diseases and Low Testosterone [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Aveed: Revolutionizing Testosterone Therapy for American Men with Low T [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Aveed: Enhancing Longevity in American Men with Hypogonadism [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Aveed: Long-Acting Testosterone Therapy for American Males with Hypogonadism [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Aveed: Long-Acting Testosterone Therapy for American Men with Hypogonadism [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
Word Count: 541