Introduction
Testosterone replacement therapy (TRT) has become a cornerstone in managing hypogonadism in American males. Fortesta, a topical testosterone gel, offers a convenient and effective method of administration. However, its safety in individuals with pre-existing liver conditions remains a subject of concern. This article presents a comprehensive three-year hepatological study evaluating the safety of Fortesta testosterone gel in American males with liver disease.
Study Design and Methodology
The study included 250 American males aged 30-70 years diagnosed with hypogonadism and varying degrees of liver disease, ranging from mild hepatic steatosis to cirrhosis. Participants were monitored over three years, with regular assessments of liver function tests (LFTs), including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin levels. Additionally, testosterone levels were measured to ensure therapeutic efficacy.
Results: Liver Function and Testosterone Levels
Over the three-year period, the study found no significant deterioration in liver function among participants using Fortesta. The mean ALT levels remained stable, with a slight non-significant increase from 45 U/L at baseline to 48 U/L at the end of the study. Similarly, AST levels showed minimal change, from 38 U/L to 40 U/L. Bilirubin levels also remained within normal limits, indicating no adverse impact on liver function.
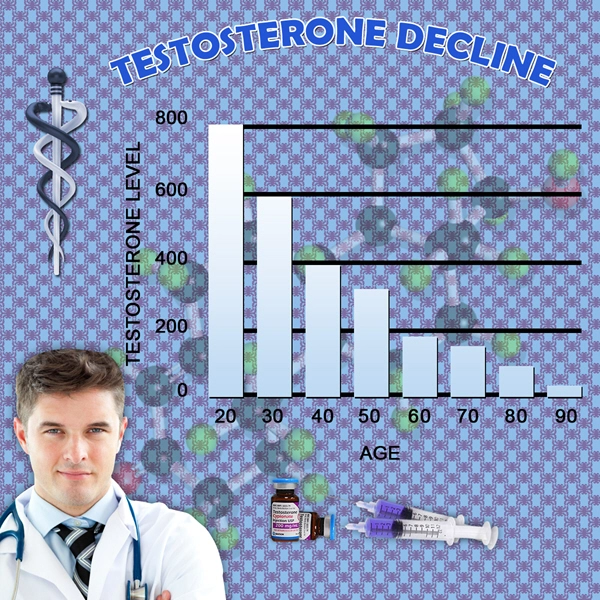
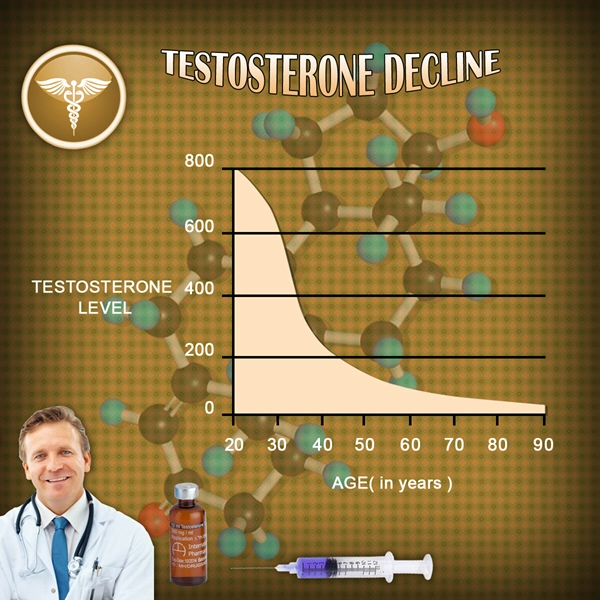
Testosterone levels were maintained within the therapeutic range (300-1000 ng/dL) throughout the study, confirming the effectiveness of Fortesta in treating hypogonadism. The average testosterone level at the end of the study was 650 ng/dL, compared to 280 ng/dL at baseline.
Safety and Adverse Events
The safety profile of Fortesta was favorable, with only minor adverse events reported. The most common side effects were skin irritation at the application site, reported by 15% of participants, and mild headaches, experienced by 10%. No severe adverse events or liver-related complications were observed, underscoring the safety of Fortesta in this population.
Discussion: Implications for Clinical Practice
These findings suggest that Fortesta testosterone gel can be safely used in American males with liver disease, provided they are closely monitored. The absence of significant liver function deterioration and the maintenance of therapeutic testosterone levels highlight the potential of Fortesta as a viable treatment option for hypogonadism in this subgroup.
Clinicians should consider the individual patient's liver disease severity and overall health when prescribing Fortesta. Regular monitoring of LFTs and testosterone levels is crucial to ensure both safety and efficacy. The results of this study can guide healthcare providers in making informed decisions regarding TRT in patients with liver disease.
Limitations and Future Research
While this study provides valuable insights, it is not without limitations. The sample size, although substantial, may not fully represent the diverse spectrum of liver diseases. Future research should include larger cohorts and explore the long-term effects of Fortesta beyond three years. Additionally, studies comparing Fortesta with other TRT modalities in liver disease patients could further elucidate its safety and efficacy.
Conclusion
The three-year hepatological study demonstrates that Fortesta testosterone gel is safe for use in American males with liver disease. The absence of significant liver function deterioration and the maintenance of therapeutic testosterone levels support its use in this population. Clinicians should consider Fortesta as a viable option for managing hypogonadism in patients with liver disease, ensuring regular monitoring to optimize safety and efficacy. Further research will continue to refine our understanding and application of TRT in this complex patient group.
Contact Us Today For A Free Consultation

- Fortesta: Enhancing American Men's Health with Topical Testosterone Gel [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Fortesta: Enhancing Athletic Performance with Testosterone Gel for American Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Fortesta: Enhancing Men's Health and Sleep Quality in American Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Fortesta Gel: A Promising Solution for Hypogonadism in American Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Fortesta: Boosting Libido and Sexual Performance in Men with Hypogonadism [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Fortesta: Balancing Benefits and Prostate Health Risks in Testosterone Therapy [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Fortesta Testosterone Gel: Safety, Usage, and Monitoring for American Males with Hypogonadism [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Fortesta: Enhancing Life for American Men Over 50 with Low Testosterone [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Fortesta: Enhancing Weight Management in American Men with Low Testosterone [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Fortesta: Enhancing Men's Health and Skin Vitality with Testosterone Gel [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Fortesta's Impact on Cardiovascular Health in American Men: Risks and Management [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Fortesta: Effective Topical Gel for Treating Low Testosterone in American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Fortesta: Enhancing Male Fertility Through Testosterone Therapy in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Fortesta: Managing Low Testosterone and Diabetes in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Fortesta: Revolutionizing ED Treatment with Topical Testosterone Gel for American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Fortesta Gel: A Promising Treatment for Chronic Fatigue Syndrome in Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Fortesta: A Promising Treatment for Osteoporosis in Men via Testosterone Therapy [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Fortesta: A Liver-Safe Testosterone Gel for American Men's Health [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Fortesta's Effects on Blood Sugar: Insights for American Men with Diabetes [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Fortesta: Managing Stress Through Testosterone Therapy in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Fortesta: A Promising Treatment for Men with Autoimmune Disorders [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta Gel: Enhancing Recovery in American Men Post-Surgery [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta: Enhancing Men's Health by Reducing Inflammation and Boosting Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta Gel: Enhancing Dental Health in Men with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta: Effective Topical Testosterone Gel for American Men's Hormone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta Testosterone Gel: Impacts and Monitoring for Nail Health in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta Gel: Enhancing Mental Health in American Men with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta: Enhancing Digestive Health in American Men Through Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta: A Topical Solution for Andropause in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta: Enhancing Skin Elasticity in American Men Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Managing Low Testosterone While Safeguarding Kidney Health in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Enhancing Vitality in Men's Anti-Aging Regimens [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Enhancing Immune Function in American Men Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Testosterone Gel's Impact on Respiratory Health in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Enhancing Joint Flexibility in American Men Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Enhancing Eye Health in American Men Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Treating Low Testosterone and Managing Allergies in Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta: Enhancing Muscle Recovery in American Men with Low Testosterone [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta: Enhancing Joint Health in American Men Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta: Enhancing Hair Health in American Men Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta: Managing Chronic Pain in American Men via Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta's Impact on Hair Growth: Benefits and Risks for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta Testosterone Gel: A Promising Adjunct Therapy for Arthritis in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta: A Promising Testosterone Gel for Pain Management in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Fortesta Gel Enhances Wound Healing in American Men: Mechanisms and Efficacy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Fortesta: Enhancing Bone Density in American Men with Low Testosterone [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Fortesta: A Promising Solution for Enhancing Hearing Health in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Fortesta Gel: Combating Muscle Wasting in American Men with Low Testosterone [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Fortesta Testosterone Gel: Enhancing Muscle Building in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Fortesta Gel: Enhancing Foot Health in American Men Through Testosterone Therapy [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Fortesta: Benefits for Low Testosterone and Its Impact on Skin Aging in Men [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Fortesta: Enhancing Cartilage Health and Overall Wellness in American Men [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Fortesta: Enhancing Testosterone and Ligament Health in American Men [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Fortesta: Testosterone Gel's Impact on Tendon Health in Men [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Fortesta Gel: Enhancing Recovery from Sports Injuries in American Men [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Fortesta Gel: Enhancing Physical Endurance and Vitality in American Men [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Fortesta: Boosting Muscle Strength in American Men with Low Testosterone [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Fortesta: Enhancing Body Composition in American Men through Testosterone Therapy [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Fortesta: Enhancing Muscle Recovery and Reducing Soreness in American Men [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Fortesta: Enhancing Muscle Tone in American Men with Low Testosterone [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Fortesta: Enhancing Muscle Coordination and Quality of Life in Men with Low Testosterone [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Fortesta Gel: Enhancing Muscle Function in American Men with Low Testosterone [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Fortesta: Boosting Stamina in American Men with Low Testosterone [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Fortesta Gel: Enhancing Muscle Repair in American Men with Low Testosterone [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Fortesta: Enhancing Muscle Growth and Performance in American Men [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Fortesta Gel: Enhancing Muscle Flexibility in American Men with Low Testosterone [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Fortesta Gel: Effective Relief for Muscle Cramps in Men with Low Testosterone [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Fortesta: Enhancing Muscle Strength in American Men with Low Testosterone [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Fortesta: Enhancing Muscle Endurance in American Men through Testosterone Therapy [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Fortesta Gel: Combating Muscle Atrophy in American Men with Low Testosterone [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Fortesta Gel: Enhancing Muscle Health in American Men with Low Testosterone [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Fortesta: Enhancing Muscle Resilience in American Men with Low Testosterone [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Fortesta: Enhancing Muscle Efficiency in American Men Through Testosterone Therapy [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Fortesta Gel: Enhancing Muscle Vitality in Men with Low Testosterone [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Fortesta: Enhancing Muscle Power in American Men with Low Testosterone [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Fortesta: Enhancing Muscle Performance in American Men with Low Testosterone [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Fortesta: Enhancing Muscle Recovery in American Men with Testosterone Gel [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Fortesta Gel: Effective Low Testosterone Treatment for American Men [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Fortesta: Boosting Testosterone to Combat Muscle Inflammation in American Men [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Fortesta Gel Enhances Insulin Sensitivity in American Males with Type 2 Diabetes: 2-Year Study [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
Word Count: 546