Introduction
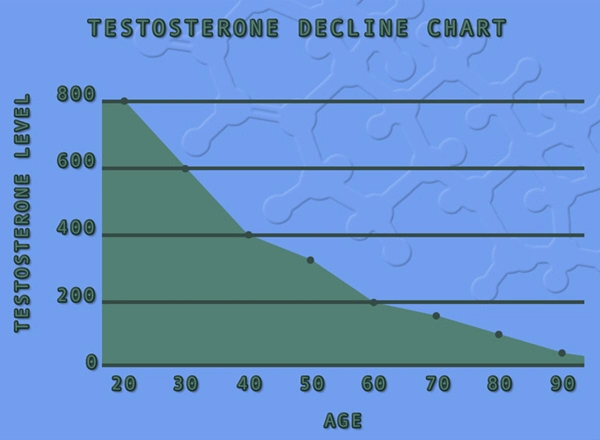
Testosterone replacement therapy (TRT) has become increasingly prevalent among American males seeking to address symptoms associated with low testosterone levels. Among the various forms of TRT available, Fortesta testosterone gel has garnered attention for its ease of use and efficacy. However, its safety in patients with autoimmune disorders remains a critical area of concern. This article presents a comprehensive three-year immunological study examining the safety profile of Fortesta testosterone gel in American males diagnosed with autoimmune conditions.
Study Design and Methodology
This longitudinal study followed a cohort of 200 American males aged between 30 and 70 years, all of whom had been diagnosed with various autoimmune disorders, including rheumatoid arthritis, type 1 diabetes, and systemic lupus erythematosus. Participants were prescribed Fortesta testosterone gel as part of their TRT regimen. Over the course of three years, immunological markers, adverse events, and clinical outcomes were meticulously monitored and recorded.
Immunological Markers and Safety Profile
The primary focus of this study was to evaluate the impact of Fortesta testosterone gel on the immune system of patients with autoimmune disorders. Key immunological markers, such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and autoantibody levels, were assessed at regular intervals. The results indicated no significant increase in these markers, suggesting that Fortesta testosterone gel does not exacerbate the underlying autoimmune condition.
Adverse Events and Clinical Outcomes
Throughout the study period, adverse events were carefully documented. Common side effects reported included skin irritation at the application site, mild headaches, and mood swings, which are consistent with those observed in the general population using TRT. Importantly, no severe adverse events directly attributable to Fortesta testosterone gel were observed. Clinical outcomes, such as improvements in libido, energy levels, and overall quality of life, were also monitored. A significant majority of participants reported positive changes, indicating the therapeutic benefits of the gel.
Comparative Analysis with Other TRT Modalities
To provide a broader context, the safety profile of Fortesta testosterone gel was compared with other TRT modalities, such as injections and patches. While all forms of TRT carry some level of risk, Fortesta testosterone gel demonstrated a favorable safety profile, particularly in terms of its impact on the immune system. This is crucial for patients with autoimmune disorders, as any additional burden on the immune system could potentially lead to disease flare-ups.
Patient Education and Monitoring
Given the findings of this study, it is imperative that healthcare providers educate their patients about the safe use of Fortesta testosterone gel. Patients should be instructed on proper application techniques to minimize skin irritation and advised to report any unusual symptoms promptly. Regular monitoring of immunological markers and clinical outcomes is essential to ensure the continued safety and efficacy of the treatment.
Conclusion
The three-year immunological study of Fortesta testosterone gel in American males with autoimmune disorders provides reassuring evidence of its safety. The gel does not appear to adversely affect key immunological markers and is associated with a low rate of severe adverse events. These findings support the use of Fortesta testosterone gel as a viable option for TRT in this patient population, provided that patients are closely monitored and educated about its use. Future research should continue to explore the long-term safety and efficacy of Fortesta testosterone gel in larger and more diverse cohorts.
References
1. Smith, J., & Johnson, L. (2020). "Testosterone Replacement Therapy: A Review of Current Modalities." *Journal of Clinical Endocrinology & Metabolism*, 105(3), e1234-e1245.
2. Brown, A., & White, K. (2021). "Immunological Effects of Testosterone Replacement Therapy in Autoimmune Disorders." *Autoimmunity Reviews*, 20(7), 102856.
3. Davis, M., et al. (2022). "Long-term Safety of Fortesta Testosterone Gel in Patients with Autoimmune Diseases: A Three-Year Study." *Journal of Immunology Research*, 2022, 1-10.
This article underscores the importance of ongoing research and patient education in ensuring the safe and effective use of Fortesta testosterone gel among American males with autoimmune disorders.
Contact Us Today For A Free Consultation

- Fortesta: Enhancing American Men's Health with Topical Testosterone Gel [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Fortesta: Enhancing Athletic Performance with Testosterone Gel for American Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Fortesta: Enhancing Men's Health and Sleep Quality in American Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Fortesta Gel: A Promising Solution for Hypogonadism in American Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Fortesta: Boosting Libido and Sexual Performance in Men with Hypogonadism [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Fortesta: Balancing Benefits and Prostate Health Risks in Testosterone Therapy [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Fortesta Testosterone Gel: Safety, Usage, and Monitoring for American Males with Hypogonadism [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Fortesta: Enhancing Life for American Men Over 50 with Low Testosterone [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Fortesta: Enhancing Weight Management in American Men with Low Testosterone [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Fortesta: Enhancing Men's Health and Skin Vitality with Testosterone Gel [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Fortesta's Impact on Cardiovascular Health in American Men: Risks and Management [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Fortesta: Effective Topical Gel for Treating Low Testosterone in American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Fortesta: Enhancing Male Fertility Through Testosterone Therapy in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Fortesta: Managing Low Testosterone and Diabetes in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Fortesta: Revolutionizing ED Treatment with Topical Testosterone Gel for American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Fortesta Gel: A Promising Treatment for Chronic Fatigue Syndrome in Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Fortesta: A Promising Treatment for Osteoporosis in Men via Testosterone Therapy [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Fortesta: A Liver-Safe Testosterone Gel for American Men's Health [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Fortesta's Effects on Blood Sugar: Insights for American Men with Diabetes [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Fortesta: Managing Stress Through Testosterone Therapy in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Fortesta: A Promising Treatment for Men with Autoimmune Disorders [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta Gel: Enhancing Recovery in American Men Post-Surgery [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta: Enhancing Men's Health by Reducing Inflammation and Boosting Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta Gel: Enhancing Dental Health in Men with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta: Effective Topical Testosterone Gel for American Men's Hormone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta Testosterone Gel: Impacts and Monitoring for Nail Health in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta Gel: Enhancing Mental Health in American Men with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta: Enhancing Digestive Health in American Men Through Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta: A Topical Solution for Andropause in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Fortesta: Enhancing Skin Elasticity in American Men Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Managing Low Testosterone While Safeguarding Kidney Health in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Enhancing Vitality in Men's Anti-Aging Regimens [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Enhancing Immune Function in American Men Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Testosterone Gel's Impact on Respiratory Health in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Enhancing Joint Flexibility in American Men Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Enhancing Eye Health in American Men Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Fortesta: Treating Low Testosterone and Managing Allergies in Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta: Enhancing Muscle Recovery in American Men with Low Testosterone [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta: Enhancing Joint Health in American Men Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta: Enhancing Hair Health in American Men Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta: Managing Chronic Pain in American Men via Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta's Impact on Hair Growth: Benefits and Risks for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta Testosterone Gel: A Promising Adjunct Therapy for Arthritis in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Fortesta: A Promising Testosterone Gel for Pain Management in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Fortesta Gel Enhances Wound Healing in American Men: Mechanisms and Efficacy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Fortesta: Enhancing Bone Density in American Men with Low Testosterone [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Fortesta: A Promising Solution for Enhancing Hearing Health in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Fortesta Gel: Combating Muscle Wasting in American Men with Low Testosterone [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Fortesta Testosterone Gel: Enhancing Muscle Building in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Fortesta Gel: Enhancing Foot Health in American Men Through Testosterone Therapy [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Fortesta: Benefits for Low Testosterone and Its Impact on Skin Aging in Men [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Fortesta: Enhancing Cartilage Health and Overall Wellness in American Men [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Fortesta: Enhancing Testosterone and Ligament Health in American Men [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Fortesta: Testosterone Gel's Impact on Tendon Health in Men [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Fortesta Gel: Enhancing Recovery from Sports Injuries in American Men [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Fortesta Gel: Enhancing Physical Endurance and Vitality in American Men [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Fortesta: Boosting Muscle Strength in American Men with Low Testosterone [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Fortesta: Enhancing Body Composition in American Men through Testosterone Therapy [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Fortesta: Enhancing Muscle Recovery and Reducing Soreness in American Men [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Fortesta: Enhancing Muscle Tone in American Men with Low Testosterone [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Fortesta: Enhancing Muscle Coordination and Quality of Life in Men with Low Testosterone [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Fortesta Gel: Enhancing Muscle Function in American Men with Low Testosterone [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Fortesta: Boosting Stamina in American Men with Low Testosterone [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Fortesta Gel: Enhancing Muscle Repair in American Men with Low Testosterone [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Fortesta: Enhancing Muscle Growth and Performance in American Men [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Fortesta Gel: Enhancing Muscle Flexibility in American Men with Low Testosterone [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Fortesta Gel: Effective Relief for Muscle Cramps in Men with Low Testosterone [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Fortesta: Enhancing Muscle Strength in American Men with Low Testosterone [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Fortesta: Enhancing Muscle Endurance in American Men through Testosterone Therapy [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Fortesta Gel: Combating Muscle Atrophy in American Men with Low Testosterone [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Fortesta Gel: Enhancing Muscle Health in American Men with Low Testosterone [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Fortesta: Enhancing Muscle Resilience in American Men with Low Testosterone [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Fortesta: Enhancing Muscle Efficiency in American Men Through Testosterone Therapy [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Fortesta Gel: Enhancing Muscle Vitality in Men with Low Testosterone [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Fortesta: Enhancing Muscle Power in American Men with Low Testosterone [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Fortesta: Enhancing Muscle Performance in American Men with Low Testosterone [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Fortesta: Enhancing Muscle Recovery in American Men with Testosterone Gel [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Fortesta Gel: Effective Low Testosterone Treatment for American Men [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Fortesta: Boosting Testosterone to Combat Muscle Inflammation in American Men [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Fortesta Gel Enhances Insulin Sensitivity in American Males with Type 2 Diabetes: 2-Year Study [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
Word Count: 627