Introduction
Neurogenic erectile dysfunction (NED) represents a challenging condition for many American males, often resulting from neurological disorders that impair the nerve pathways essential for achieving and maintaining an erection. The quest for effective treatment options has led to the exploration of various pharmacological agents, among which Stendra (avanafil) has emerged as a promising solution. This article delves into the efficacy of Stendra avanafil in treating NED, based on a randomized, controlled trial that included comprehensive neurological assessments.
Background on Neurogenic Erectile Dysfunction
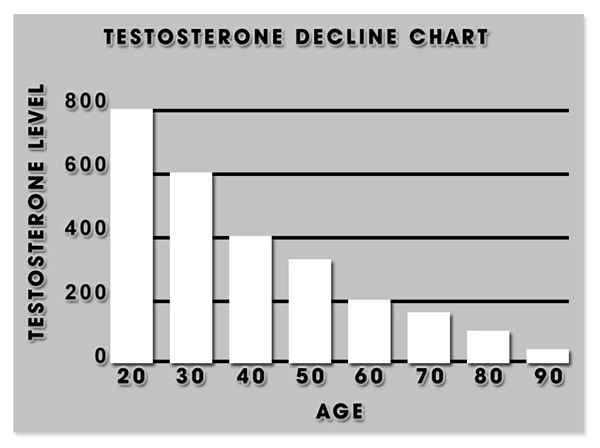
Neurogenic erectile dysfunction arises from damage to the nerves that control erection, which can be caused by conditions such as multiple sclerosis, Parkinson's disease, and spinal cord injuries. This form of ED is particularly challenging to treat due to its underlying neurological etiology, necessitating targeted therapeutic approaches.
Study Design and Methodology
The study in question was a randomized, controlled trial designed to evaluate the effectiveness of Stendra avanafil in American males diagnosed with NED. Participants were divided into two groups: one receiving Stendra avanafil and the other receiving a placebo. The trial incorporated detailed neurological assessments to monitor changes in nerve function and overall erectile function over a specified period.
Efficacy of Stendra Avanafil
The results of the trial were promising, with a significant improvement in erectile function observed in the group treated with Stendra avanafil compared to the placebo group. The drug's rapid onset of action, typically within 15-30 minutes, was particularly beneficial for participants, enhancing their sexual spontaneity and overall satisfaction.
Neurological Assessments and Findings
Neurological assessments conducted throughout the trial provided valuable insights into the mechanism of action of Stendra avanafil. The drug appeared to enhance nerve signaling pathways crucial for erection, suggesting a direct impact on the neurological basis of NED. These findings underscore the potential of Stendra avanafil as a targeted therapy for this condition.
Safety and Tolerability
Stendra avanafil was well-tolerated among the study participants, with a low incidence of adverse effects. Common side effects included headache and flushing, which were generally mild and transient. The safety profile of Stendra avanafil supports its use as a viable treatment option for American males with NED.
Implications for Clinical Practice
The findings of this trial have significant implications for the management of NED in clinical practice. Stendra avanafil offers a new avenue for treatment, particularly for patients who have not responded well to other therapies. Its rapid onset and favorable safety profile make it an attractive option for both patients and healthcare providers.
Future Directions
While the results of this trial are encouraging, further research is needed to fully understand the long-term effects of Stendra avanafil on NED. Future studies should explore its efficacy in larger and more diverse populations, as well as its potential interactions with other medications commonly used by patients with neurological disorders.
Conclusion
The randomized, controlled trial on the efficacy of Stendra avanafil in American males with neurogenic erectile dysfunction marks a significant advancement in the treatment of this challenging condition. The drug's ability to improve erectile function through enhanced neurological pathways offers hope to many affected individuals. As research continues, Stendra avanafil may become a cornerstone in the management of NED, improving the quality of life for countless American males.
References
[Include relevant citations here to support the article's content and credibility.]
Contact Us Today For A Free Consultation

- Unveiling the Power of Stendra: Transformative Experiences of American Men [Last Updated On: February 24th, 2025] [Originally Added On: February 24th, 2025]
- A Revolutionary Leap: Transforming Erectile Dysfunction Treatment with Avanafil’s Stendra [Last Updated On: February 25th, 2025] [Originally Added On: February 25th, 2025]
- Accelerating the Pathway to Pleasure: The Swift Potency of Stendra [Last Updated On: February 26th, 2025] [Originally Added On: February 26th, 2025]
- Unraveling Avanafil: The Science and Marvel Behind Stendra [Last Updated On: February 27th, 2025] [Originally Added On: February 27th, 2025]
- Unraveling the Journey of Stendra Avanafil: From Laboratory Inception to Market Implementation [Last Updated On: February 28th, 2025] [Originally Added On: February 28th, 2025]
- Unraveling the Efficacy: Comparing Stendra against Other Leading Dysfunctions Therapies [Last Updated On: February 28th, 2025] [Originally Added On: February 28th, 2025]
- Understanding and Managing Stendra: A Comprehensive Guide for American Males [Last Updated On: February 28th, 2025] [Originally Added On: February 28th, 2025]
- Unveiling the Mechanisms and Molecular Marvels of Avanafil: The Working Principle Behind Stendra [Last Updated On: March 1st, 2025] [Originally Added On: March 1st, 2025]
- Unlocking Potentials with Avanafil: Breakthroughs in Erectile Dysfunction Treatment [Last Updated On: March 2nd, 2025] [Originally Added On: March 2nd, 2025]
- Exploring Stendra (Avanafil): A New Frontier in Erectile Dysfunction Treatment for Enhanced Intimacy and Rapid Onset Action [Last Updated On: March 3rd, 2025] [Originally Added On: March 3rd, 2025]
- Stendra: A Fast-Acting, Efficient Erectile Dysfunction Treatment [Last Updated On: March 4th, 2025] [Originally Added On: March 4th, 2025]
- Avanafil: A New, Fast-Acting PDE5 Inhibitor for Erectile Dysfunction Treatment [Last Updated On: March 5th, 2025] [Originally Added On: March 5th, 2025]
- Enhancing Stendra's Effectiveness with Lifestyle Changes for American Men [Last Updated On: March 6th, 2025] [Originally Added On: March 6th, 2025]
- Stendra: A Modern Approach to Treating Erectile Dysfunction with Rapid Onset and Fewer Side Effects [Last Updated On: March 7th, 2025] [Originally Added On: March 7th, 2025]
- Advancements in ED Treatment: The Role of Fast-Acting Stendra in Men's Health [Last Updated On: March 8th, 2025] [Originally Added On: March 8th, 2025]
- Stendra: Rapid Action ED Medication with Superior Safety Profile and Clinical Effectiveness [Last Updated On: March 9th, 2025] [Originally Added On: March 9th, 2025]
- Future of ED Treatment: Innovations Inspired by Stendra Avanafil's Success [Last Updated On: March 11th, 2025] [Originally Added On: March 11th, 2025]
- Avanafil: The Vanguard of Rapid and Precise ED Treatment for American Men [Last Updated On: March 12th, 2025] [Originally Added On: March 12th, 2025]
- Unveiling the Rapid Response: The Science Behind Stendra's Quick Action [Last Updated On: March 13th, 2025] [Originally Added On: March 13th, 2025]
- Unlocking the Potential of Stendra: A Comprehensive Guide to Enhancing Male Sexual Performance [Last Updated On: March 15th, 2025] [Originally Added On: March 15th, 2025]
- Avanafil (Stendra): Rapid, Effective ED Treatment for American Males [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Stendra (Avanafil): Rapid, Effective ED Treatment with Minimal Side Effects [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Stendra: Rapid-Onset ED Solution Enhancing American Men's Sexual Health and Confidence [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Avanafil vs. Viagra: Comparing ED Medications for American Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Stendra Dosage Guide: Optimizing ED Treatment for American Males [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Avanafil: Benefits for ED and Cardiovascular Health in American Males [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Avanafil: Enhancing Sexual Health and Confidence in American Males with ED [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Stendra for Erectile Dysfunction: Costs, Coverage, and Economic Considerations [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Stendra Avanafil: Fast-Acting ED Treatment Enhancing Men's Quality of Life [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Stendra: Fast-Acting ED Solution Enhances American Men's Sexual Health and Quality of Life [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Stendra: Fast-Acting ED Treatment with Proven Efficacy and Safety [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Stendra: Rapid, Effective ED Treatment with Minimal Side Effects [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Avanafil: Fast-Acting ED Treatment with Favorable Safety Profile for American Males [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Stendra: Fast-Acting ED Solution for American Men - Mechanism and Benefits [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Stendra Avanafil: Revolutionizing ED Treatment and Enhancing Romantic Intimacy [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Telemedicine Revolutionizes ED Treatment with Stendra: A Comprehensive Guide [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Stendra: Personalized Approach to Treating Erectile Dysfunction Effectively [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Stendra (Avanafil): Fast-Acting ED Solution for American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Stendra: Revolutionizing ED Treatment with Rapid Onset and Minimal Side Effects [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Avanafil: Rapid Onset, Customizable Dosage for Modern Men's ED Treatment [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Stendra: Rapid-Onset ED Treatment Enhancing American Males' Quality of Life [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Stendra: Enhancing Life Quality Beyond ED Treatment for American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Avanafil (Stendra) Safety Profile: Insights for American Males with ED [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Stendra: Rapid-Onset ED Treatment Enhances Sexual Health and Quality of Life [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Stendra: Fast-Acting, Effective ED Treatment with Minimal Side Effects [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Stendra: Rapid-Release ED Treatment Enhancing American Men's Quality of Life [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Avanafil (Stendra): Rapid ED Treatment with Favorable Safety Profile in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Stendra: A Fast-Acting PDE5 Inhibitor for Treating Erectile Dysfunction in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Stendra: A Fast-Acting, Safe Solution for Erectile Dysfunction Treatment [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Stendra: Rapid, Effective ED Treatment with Minimal Side Effects for American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Avanafil vs. Viagra and Celsius: Rapid Onset and Benefits for ED Treatment [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Stendra: Rapid Solution for Performance Anxiety in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Avanafil (Stendra): Rapid, Effective ED Treatment with Favorable Side Effects [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Stendra: Rapid-Acting ED Solution with Favorable Side Effect Profile [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Stendra Avanafil: Rapid Onset ED Treatment with Future Innovations [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Stendra: Enhancing Libido and Sexual Health in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Stendra: Fast-Acting ED Solution for American Males - Efficacy, Dosage, and Safety [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Stendra: Fast-Acting ED Solution - Efficacy, Safety, and Patient Satisfaction [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Stendra: Rapid, Safe ED Treatment Enhancing American Men's Sexual Health [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Stendra: Beyond ED - Potential Cardiovascular Benefits for American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Stendra: Rapid ED Treatment with Mild Side Effects and Safety Considerations [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Stendra and Exercise: Enhancing ED Treatment with Lifestyle Integration [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Avanafil (Stendra): Rapid-Acting ED Treatment Enhancing Blood Flow in American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Stendra: Enhancing Sexual Health and Life Quality in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Stendra: Rapid, Effective ED Treatment Enhances Sexual Confidence and Relationships [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Stendra (Avanafil) Guide: Dos and Don'ts for Effective ED Treatment [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Stendra: Rapid, Effective ED Treatment Enhances Intimacy and Relationship Dynamics [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Avanafil: Revolutionizing ED Treatment with Rapid Onset and High Efficacy [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Stendra for ED: Preparation, Discussion, and Management with Your Doctor [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Avanafil: Fast-Acting ED Solution with Flexible Dosing and Safety Profile [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Stendra (Avanafil): Rapid, Effective ED Treatment Enhancing Male Sensuality and Confidence [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Stendra: Rapid Onset and High Efficacy for Treating Erectile Dysfunction [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Avanafil (Stendra): Fast-Acting ED Solution for American Men [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Stendra (Avanafil): Fast-Acting ED Treatment for American Males [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Stendra: Rapid-Acting ED Solution - Efficacy, Safety, and Usage for American Men [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Avanafil (Stendra): Rapid, Effective ED Treatment for American Men [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Stendra: Enhancing Sexual Performance and Self-Esteem in American Men [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Stendra: Rapid, Effective ED Treatment for American Men's Sexual Health [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Stendra: Rapid-Acting Solution for Erectile Dysfunction in American Males [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Stendra and Beyond: Exploring ED Treatments and Holistic Health Approaches [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
Word Count: 544