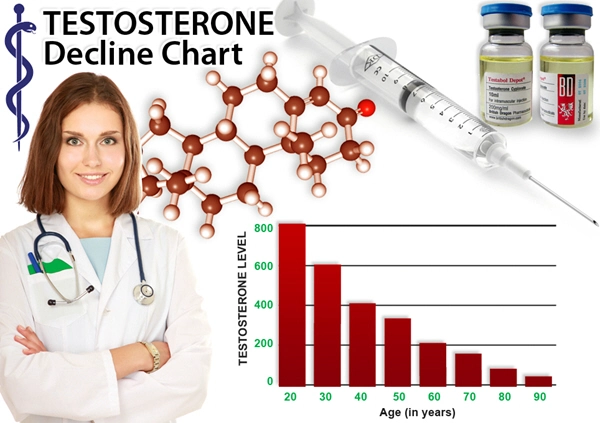
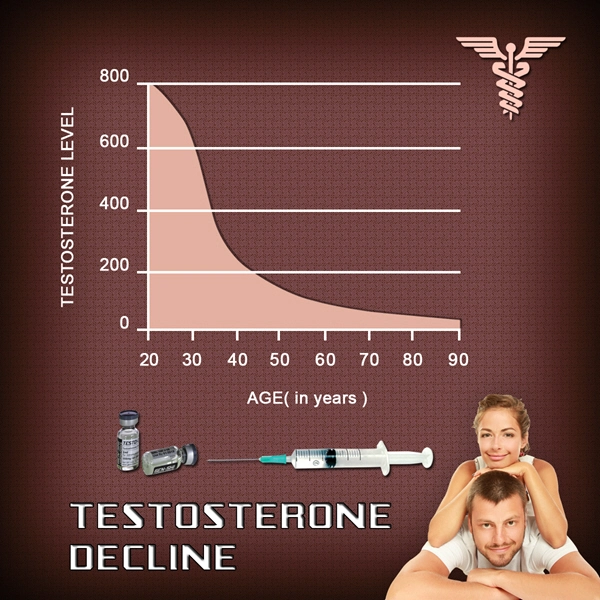
Introduction
Type 2 diabetes mellitus (T2DM) represents a significant health challenge in the United States, particularly among American males, where it is associated with reduced insulin sensitivity and increased morbidity. The Striant testosterone buccal system, a novel form of testosterone replacement therapy, has been investigated for its potential to improve insulin sensitivity in this population. This article explores the findings of a randomized controlled trial that assessed the efficacy of the Striant system in enhancing insulin sensitivity among American males with T2DM.
Study Design and Methodology
The study was a randomized, double-blind, placebo-controlled trial involving 150 American males aged 40 to 70 years with diagnosed T2DM and low testosterone levels. Participants were randomly assigned to receive either the Striant testosterone buccal system or a placebo for a period of 12 weeks. Insulin sensitivity was assessed using the hyperinsulinemic-euglycemic clamp technique, a gold standard for measuring insulin action in vivo, at baseline and at the end of the treatment period.
Results of the Trial
The results of the trial demonstrated a significant improvement in insulin sensitivity among the group treated with the Striant testosterone buccal system compared to the placebo group. Specifically, the mean increase in glucose disposal rate, a measure of insulin sensitivity, was 22% higher in the Striant group (p < 0.01). Additionally, participants in the Striant group experienced a modest but statistically significant reduction in HbA1c levels, indicating better long-term glucose control.
Mechanisms of Action
The enhancement of insulin sensitivity by the Striant testosterone buccal system can be attributed to several mechanisms. Testosterone is known to influence glucose metabolism by increasing muscle mass, which in turn increases the capacity for glucose uptake. Furthermore, testosterone may directly affect insulin signaling pathways, improving the body's response to insulin. The buccal delivery system ensures a steady release of testosterone, mimicking the body's natural circadian rhythm, which may contribute to its efficacy in improving insulin sensitivity.
Clinical Implications
The findings of this trial have significant clinical implications for the management of T2DM in American males with low testosterone levels. The use of the Striant testosterone buccal system could offer a dual benefit of improving both testosterone deficiency and insulin sensitivity, potentially reducing the risk of diabetes-related complications. Clinicians should consider testosterone levels in their male patients with T2DM and discuss the potential benefits of testosterone replacement therapy as part of a comprehensive treatment plan.
Safety and Side Effects
The trial also monitored the safety profile of the Striant testosterone buccal system. The most commonly reported side effects were mild and included gum irritation and bitter taste, which were transient and resolved with continued use. No serious adverse events were reported, suggesting that the Striant system is well-tolerated among American males with T2DM.
Conclusion
The Striant testosterone buccal system represents a promising therapeutic option for improving insulin sensitivity in American males with T2DM and low testosterone levels. The results of this randomized controlled trial provide evidence of its efficacy and safety, highlighting its potential role in the management of this prevalent condition. Further studies are warranted to explore the long-term benefits and to optimize the integration of testosterone replacement therapy in the treatment of T2DM.
Future Directions
Future research should focus on larger, long-term studies to confirm the sustained benefits of the Striant testosterone buccal system on insulin sensitivity and glycemic control. Additionally, investigating the impact of this therapy on other diabetes-related outcomes, such as cardiovascular risk and quality of life, would provide a more comprehensive understanding of its role in the management of T2DM in American males.
Contact Us Today For A Free Consultation

- Striant: Testosterone Buccal System - Side Effects, Safety, and Monitoring for American Males [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Striant: Innovative Buccal Testosterone Therapy for Young American Males with Hypogonadism [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Striant: Enhancing Vitality in Aging American Men Through Testosterone Therapy [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Striant: Innovative Buccal Testosterone Therapy for American Men with Hypogonadism [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Striant: Innovative Buccal System for Testosterone Replacement in American Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Striant: Enhancing Muscle Growth and Fat Loss in American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Striant Therapy: Monitoring Testosterone, Hematocrit, and Prostate Health in American Men [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Striant: Innovative Buccal Testosterone Therapy for American Men's Health [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Striant: A Convenient Buccal Solution for American Men's Testosterone Therapy [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Striant: Enhancing Mood and Cognitive Function in American Men via Testosterone Therapy [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Striant: Innovative Buccal System Boosts Energy in Men with Low Testosterone [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Striant: A Buccal Testosterone Solution for Muscle Wasting in American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Striant and Diabetes: Managing Testosterone and Blood Sugar in American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Striant Buccal System: Optimizing Testosterone Therapy for American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Striant: Non-Invasive TRT Option for American Men with Hypogonadism [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Striant: Empowering American Men Against Age-Related Testosterone Decline [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Striant: Effective Testosterone Therapy for Andropause in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Striant: Enhancing Sleep Quality in American Males with Testosterone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Striant: Enhancing Life Post-Prostatectomy with Buccal Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Revolutionizing Testosterone Therapy for American Males' Sexual Health [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Enhancing Bone Health in American Men with Low Testosterone [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Enhancing Vision and Eye Health in American Men Through Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Enhancing Immune Health in American Men via Testosterone Replacement Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Enhancing Respiratory Health in American Men via Buccal Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant's Effects on Male Fertility and Reproductive Health: A Comprehensive Overview [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Enhancing Athletic Performance and Recovery in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Enhancing Health in Men with Metabolic Syndrome Through Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Striant: Enhancing Stress Management and Resilience in American Men with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Striant: Enhancing Men's Kidney and Urinary Health Through Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Striant: Enhancing Men's Liver Health via Buccal Testosterone Delivery [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Striant: A Novel Adjunct in Managing Male Pattern Baldness [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Striant Buccal System: Effective Testosterone Therapy for Hypogonadism in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Striant: A Lifeline for American Men Battling Chronic Fatigue from Low Testosterone [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant: Advancing Testosterone Therapy for American Men's Health and Vitality [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant: Revolutionizing Testosterone Therapy for American Males with Hypogonadism [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant: Enhancing Mental Health in American Men with Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant: Innovative Buccal System for Testosterone Deficiency in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant Buccal System: Effective Testosterone Therapy for American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant: Enhancing Cardiovascular Health in American Men with Hypogonadism [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant: Testosterone Therapy's Potential in Enhancing Men's Auditory Health [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Striant: Revolutionizing Testosterone Therapy for American Men's Hormonal Health [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Striant: Enhancing Digestive Health in American Men Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Striant: Advancing Men's Health with Buccal Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Striant: Enhancing Fertility in American Men with Testosterone Deficiency [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Striant: Revolutionizing Men's Skin Health with Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Striant: Enhancing Joint Health and Mobility in American Men with Low Testosterone [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Striant: Testosterone Therapy's Impact on Dental Health and Oral Care Strategies [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Striant: Enhancing Weight Management in American Men via Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Striant: Enhancing Sleep Quality and Patterns in American Men with Hypogonadism [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Striant: Enhancing Musculoskeletal Health in American Men Through Testosterone Therapy [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Striant: Enhancing Emotional Well-being in American Men with Hypogonadism [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Striant Buccal System: Enhancing Cognitive Health in American Men [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Striant: Enhancing Male Endurance and Stamina through Innovative Testosterone Therapy [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Striant: Enhancing Immune Health and Disease Prevention in American Men [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Striant: Enhancing Bone Health in American Men Through Testosterone Therapy [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Striant: Enhancing Male Sexual Health and Vitality with Buccal Testosterone Therapy [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Striant: Enhancing American Men's Health Through Testosterone Buccal Therapy [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Striant: Enhancing Cardiovascular Fitness and Heart Health in American Men [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Striant: Enhancing Muscle Growth and Recovery in American Men with Hypogonadism [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Striant: Enhancing Vision and Eye Health in American Men Through Testosterone Therapy [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Striant: A Breakthrough in Testosterone Therapy for Hair Growth in American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Striant: Enhancing Testosterone Therapy and Oral Health in American Men [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Striant: A Novel Approach to Prostate Health and Cancer Prevention in American Men [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Striant's Role in Enhancing Joint Health for American Men with Arthritis [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Striant: Enhancing Digestive Efficiency and Gut Health in American Men [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Striant: Enhancing Emotional Resilience and Reducing Stress in Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Striant: Enhancing Hearing Acuity in American Men Through Testosterone Therapy [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Striant: Enhancing Liver Health and Detoxification in American Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Striant: Enhancing Testosterone and Managing Sperm Production in American Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Striant: Enhancing Sleep and Circadian Rhythms in American Men via Testosterone Therapy [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Striant: Enhancing Musculoskeletal Strength and Flexibility in American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Striant: Enhancing Skin Elasticity and Appearance in American Men [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Striant: Enhancing Kidney Function and Urinary Health in American Men [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Striant: Enhancing Cognitive Health in American Men Through Testosterone Therapy [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Striant: Enhancing Respiratory Health in American Men with Testosterone Therapy [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Striant: A Novel Buccal System for Testosterone Replacement in American Men [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Striant: A Novel Buccal System for Testosterone Replacement in American Men [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Striant: Enhancing Men's Emotional Health Through Testosterone Therapy [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Striant: Enhancing Cognitive Function through Buccal Testosterone Delivery in American Men [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Striant: Convenient Buccal Testosterone Therapy for American Men with Hypogonadism [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
Word Count: 587