Class: Androgens Note: This monograph also contains information on Testosterone Cypionate, Testosterone Enanthate VA Class: HS100 CAS Number: 58-22-0 Brands: Androderm, AndroGel, Delatestryl, Striant, Testim
Special Alerts:
[Posted 01/31/2014] ISSUE: FDA is investigating the risk of stroke, heart attack, and death in men taking FDA-approved testosterone products. We have been monitoring this risk and decided to reassess this safety issue based on the recent publication of two separate studies that each suggested an increased risk of cardiovascular events among groups of men prescribed testosterone therapy. FDA is providing this alert while it continues to evaluate the information from these studies and other available data. FDA will communicate final conclusions and recommendations when the evaluation is complete.
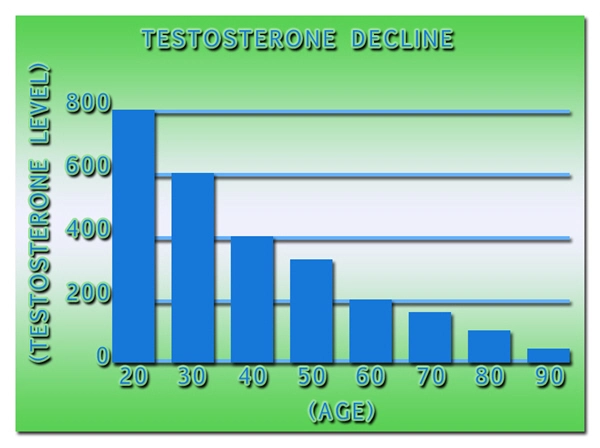
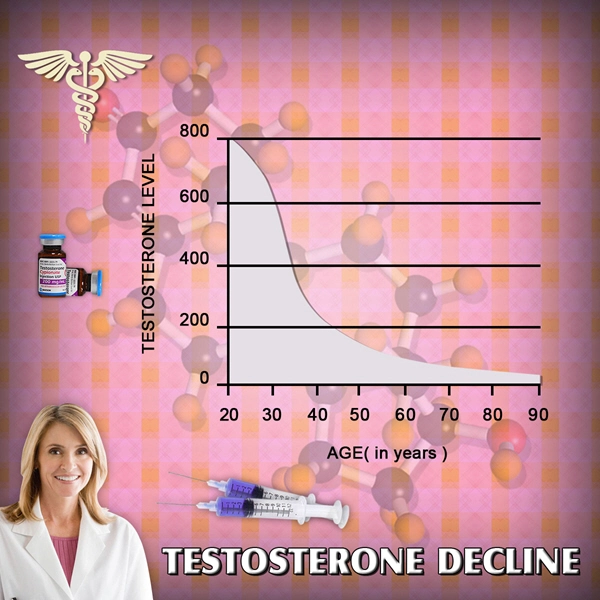
BACKGROUND: Testosterone is a hormone essential to the development of male growth and masculine characteristics. Testosterone products are FDA-approved only for use in men who lack or have low testosterone levels in conjunction with an associated medical condition.
RECOMMENDATION: At this time, FDA has not concluded that FDA-approved testosterone treatment increases the risk of stroke, heart attack, or death. Patients should not stop taking prescribed testosterone products without first discussing any questions or concerns with their health care professionals. Health care professionals should consider whether the benefits of FDA-approved testosterone treatment is likely to exceed the potential risks of treatment. The prescribing information in the drug labels of FDA-approved testosterone products should be followed.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program.
For more information visit the FDA website at: and .
REMS:
FDA approved a REMS for testosterone to ensure that the benefits of a drug outweigh the risks. The REMS may apply to one or more preparations of testosterone and consists of the following: medication guide. See the FDA REMS page () or the ASHP REMS Resource Center ().
Risk of virilization in children and women following secondary exposure to testosterone in topically administered testosterone gel.157 166 170 171 Advise children and women to avoid contact with application sites of men using testosterone gel.166 170 171 (See Virilization in Children and Women from Secondary Exposure to Testosterone under Cautions.)
Read more from the original source:
Testosterone Monograph for Professionals - Drugs.com
Contact Us Today For A Free Consultation

- New female 'sex-drive' drug to be tested [Last Updated On: January 14th, 2025] [Originally Added On: November 2nd, 2012]
- BioSante will merge with ANI Pharma in stock deal [Last Updated On: December 13th, 2017] [Originally Added On: November 2nd, 2012]
- Female sex-enhancing nasal spray undergoing clinical trials [Last Updated On: October 24th, 2015] [Originally Added On: November 2nd, 2012]
- Project 47 XXY - Living with Klinefelter's Syndrome - Video [Last Updated On: January 15th, 2025] [Originally Added On: November 2nd, 2012]
- The Benefits of Testosterone Gel - Video [Last Updated On: January 16th, 2025] [Originally Added On: November 2nd, 2012]
- Pros And Cons of Testim - Video [Last Updated On: January 15th, 2025] [Originally Added On: November 2nd, 2012]
- BioSante Pharma Completes Enrollment in Pivotal LibiGel Trials - Video [Last Updated On: January 16th, 2025] [Originally Added On: November 2nd, 2012]
- Testosterone Replacement and Sperm Production - Video [Last Updated On: January 17th, 2025] [Originally Added On: November 2nd, 2012]
- Androgel July 09, 2010, 02:32 PM - Video [Last Updated On: January 17th, 2025] [Originally Added On: November 2nd, 2012]
- Insidermedicine In Depth - July 8, 2010 - Testosterone - Video [Last Updated On: January 18th, 2025] [Originally Added On: November 2nd, 2012]
- A 'female Viagra' nasal spray has started clinical trials [Last Updated On: May 31st, 2025] [Originally Added On: November 9th, 2012]
- GTA IV Heliride - Standing Erection - Video [Last Updated On: December 10th, 2012] [Originally Added On: December 10th, 2012]
- Testosterone gels can affect women, kids [Last Updated On: December 22nd, 2017] [Originally Added On: January 14th, 2013]
- Questions Answers - Detox And Testosterone Gel [Last Updated On: December 29th, 2016] [Originally Added On: August 16th, 2013]
- Testosterone gel: Indications, Side Effects, Warnings - Drugs.com [Last Updated On: December 1st, 2017] [Originally Added On: October 31st, 2013]
- AndroGel® (testosterone gel) 1% - National Institutes of Health [Last Updated On: December 21st, 2017] [Originally Added On: October 31st, 2013]
- What is Testosterone Gel? | Consumer Health PanelConsumer Health Panel [Last Updated On: December 27th, 2017] [Originally Added On: November 15th, 2013]
- What is Testosterone Gel? | Consumer Health PanelConsumer ... [Last Updated On: January 18th, 2018] [Originally Added On: November 18th, 2013]
- AndroGel testosterone gel New FDA Drug Approval | CenterWatch [Last Updated On: January 4th, 2018] [Originally Added On: November 23rd, 2013]
- Testosterone skin gel: Information from Answers.com [Last Updated On: April 1st, 2025] [Originally Added On: November 28th, 2013]
- Testosterone Gels | Do They Work? [Last Updated On: March 28th, 2020] [Originally Added On: December 5th, 2013]
- Testosterone Gel Treatment | eHow - eHow | How to Videos ... [Last Updated On: December 25th, 2017] [Originally Added On: December 6th, 2013]
- Side Effects of AndroGel (Testosterone Gel for Topical Use ... [Last Updated On: December 2nd, 2017] [Originally Added On: December 10th, 2013]
- Female Sexual Desire Drug Rejected by U.S. Regulators [Last Updated On: January 30th, 2018] [Originally Added On: December 12th, 2013]
- Manufacturers of "female Viagra" appeal FDA denial [Last Updated On: December 26th, 2017] [Originally Added On: December 12th, 2013]
- AndroGel (Testosterone Gel for Topical Use) Drug Information ... [Last Updated On: January 23rd, 2018] [Originally Added On: December 24th, 2013]
- Testosterone Gel Vs. Patch | eHow - eHow | How to Videos ... [Last Updated On: December 3rd, 2017] [Originally Added On: December 24th, 2013]
- Testosterone gel: Indications, Side Effects, Warnings ... [Last Updated On: December 18th, 2017] [Originally Added On: January 23rd, 2014]
- Testosterone Treatments Linked to Heart Attacks [Last Updated On: November 28th, 2020] [Originally Added On: January 30th, 2014]
- Testosterone Tx Ups Heart Attack Risk at Any Age [Last Updated On: November 4th, 2020] [Originally Added On: January 31st, 2014]
- Questions & Answers 195 - Video [Last Updated On: November 14th, 2020] [Originally Added On: February 5th, 2014]
- Startling Effects of Testosterone Therapy [Last Updated On: October 29th, 2020] [Originally Added On: February 8th, 2014]
- Group wants heart attack warning on testosterone - Quincy Herald-Whig | Illinois & Missouri News, Sports [Last Updated On: October 16th, 2020] [Originally Added On: February 25th, 2014]
- Could the male hormone testosterone transform a woman's looks, life and libido? [Last Updated On: October 21st, 2020] [Originally Added On: March 6th, 2014]
- Perrigo sues FDA over failure to grant therapeutic equivalence rating for testosterone gel [Last Updated On: October 3rd, 2020] [Originally Added On: March 24th, 2014]
- Low T Litigation - Video [Last Updated On: October 15th, 2020] [Originally Added On: April 2nd, 2014]
- Testosterone Cypionate | Best Testosterone Supplments [Last Updated On: October 15th, 2020] [Originally Added On: April 14th, 2014]
- Is Your Husband At Risk for a Testosterone Heart Attack? [Last Updated On: October 26th, 2020] [Originally Added On: April 16th, 2014]
- How to Apply Testosterone Gel - 31 Day Testosterone Plan - Video [Last Updated On: November 15th, 2020] [Originally Added On: April 22nd, 2014]
- AUXL Gives Bleak Outlook, IG Reverses Loss, ENDP Opens Wallet, BOTA Stops Work [Last Updated On: October 22nd, 2020] [Originally Added On: April 30th, 2014]
- Andractim Testosterone Gel for Testosterone Replacement ... [Last Updated On: October 13th, 2020] [Originally Added On: April 30th, 2014]
- Testosterone Gels [Last Updated On: December 27th, 2017] [Originally Added On: May 1st, 2014]
- Double Blessing For Trimel Pharma... [Last Updated On: May 4th, 2015] [Originally Added On: May 31st, 2014]
- Book Extract: Set ambitious targets to win [Last Updated On: November 7th, 2020] [Originally Added On: June 2nd, 2014]
- Testosterone Nasal Gel Claims To Help Women Reach Orgasm (And Has 'No Side Effects') [Last Updated On: November 30th, 2020] [Originally Added On: June 18th, 2014]
- Wounded veteran is standing tall, looking to the future [Last Updated On: October 6th, 2020] [Originally Added On: July 6th, 2014]
- Wonkblog: Britains economy is finally bigger than it was in 2008. What took so long? [Last Updated On: October 21st, 2020] [Originally Added On: July 26th, 2014]
- As Political Disenchantment Soars, Lines At The Polls Grow Shorter [Last Updated On: November 10th, 2020] [Originally Added On: July 26th, 2014]
- Love Letters: We Don't Have Much To Talk About [Last Updated On: October 28th, 2020] [Originally Added On: July 26th, 2014]
- Why Isn't This Swirling Low in the Atlantic a Tropical Storm? [Last Updated On: November 12th, 2020] [Originally Added On: August 2nd, 2014]
- Don't Make This Big Investing Mistake Once Interest Rates Start Rising [Last Updated On: November 23rd, 2020] [Originally Added On: August 2nd, 2014]
- 'When I look at myself I am not impressed at all' [Last Updated On: October 11th, 2020] [Originally Added On: August 2nd, 2014]
- Androxal superior to Androgel in Phase 3 trial [Last Updated On: October 17th, 2020] [Originally Added On: September 27th, 2014]
- Woman Says Dentists Testosterone Gel Rubbed Off On Her During Sexual Attack, Causing Her To Grow Unsightly Body Hair [Last Updated On: November 13th, 2020] [Originally Added On: September 30th, 2014]
- testosterone gel (Androgel): Drug Facts, Side Effects and ... [Last Updated On: November 16th, 2020] [Originally Added On: October 3rd, 2014]
- Buy Cernos Gel, Buy Testosterone Gel from Certified Online ... [Last Updated On: April 26th, 2025] [Originally Added On: October 28th, 2014]
- AbbVie Sales Blow Past Expectations [Last Updated On: November 30th, 2020] [Originally Added On: October 31st, 2014]
- What Are the Benefits of Testosterone Gel? | eHow [Last Updated On: November 3rd, 2020] [Originally Added On: November 2nd, 2014]
- Secaucus police officer charged with selling steroid gel on eBay: officials [Last Updated On: October 31st, 2020] [Originally Added On: November 18th, 2014]
- Endo Int'l Buys Rights To Natesto Testosterone Nasal Gel From Trimel BioPharma [Last Updated On: October 1st, 2020] [Originally Added On: November 25th, 2014]
- DailyMed - ANDROGEL- testosterone gel [Last Updated On: January 3rd, 2018] [Originally Added On: November 25th, 2014]
- Doctor's Testosterone Gel - Buy Doctors Testosterone Gel ... [Last Updated On: November 24th, 2020] [Originally Added On: December 28th, 2014]
- Testosterone Gel A Convenient, Natural Solution [Last Updated On: October 24th, 2015] [Originally Added On: December 30th, 2014]
- Gilead rival AbbVie's new hepatitis C treatment wins EU approval [Last Updated On: November 28th, 2020] [Originally Added On: January 20th, 2015]
- Men, women: Why cholesterol matters [Last Updated On: October 28th, 2020] [Originally Added On: January 30th, 2015]
- Vital Health, Inc. Provides Tips On How To Combat Cystic Acne Through Dietary Changes and Natural Remedies [Last Updated On: May 4th, 2015] [Originally Added On: February 20th, 2015]
- Higher heart disease risk for men due to high testosterone, low estrogen [Last Updated On: May 4th, 2015] [Originally Added On: March 9th, 2015]
- Men's heart disease risk linked to high testosterone and low estrogen [Last Updated On: November 27th, 2020] [Originally Added On: March 9th, 2015]
- High testosterone ups heart disease risk in men [Last Updated On: May 4th, 2015] [Originally Added On: March 10th, 2015]
- Testosterone Gel - How RS Transaderm makes your test ... [Last Updated On: November 15th, 2020] [Originally Added On: March 12th, 2015]
- Low T Treatment | FORTESTA (testosterone) Gel CIII [Last Updated On: October 19th, 2020] [Originally Added On: March 17th, 2015]
- Why Repros Therapeutics, Inc. Shares Are Soaring [Last Updated On: October 5th, 2020] [Originally Added On: April 2nd, 2015]
- Testosterone Gel Treatment of Gynecomastia | eHow [Last Updated On: January 1st, 2018] [Originally Added On: April 6th, 2015]
- DailyMed - TESTOSTERONE- testosterone gel, metered [Last Updated On: October 22nd, 2020] [Originally Added On: July 3rd, 2015]
- Testim gel: Indications, Side Effects, Warnings - Drugs.com [Last Updated On: October 19th, 2020] [Originally Added On: July 3rd, 2015]
- Testim (Testosterone Gel) Drug Information: Description ... [Last Updated On: October 25th, 2020] [Originally Added On: July 3rd, 2015]
- AndroGel® (Testosterone Gel) 1.62% [Last Updated On: December 11th, 2017] [Originally Added On: August 2nd, 2015]
- AndroGel (testosterone gel) [Last Updated On: December 28th, 2017] [Originally Added On: August 12th, 2015]
- List Of Low-Carb Doctors [Last Updated On: October 10th, 2020] [Originally Added On: September 25th, 2015]
- Low Thyroid Level Symptoms | HagglundMD.com Thyroid Doctor [Last Updated On: October 6th, 2020] [Originally Added On: September 26th, 2015]
Word Count: 375