Introduction
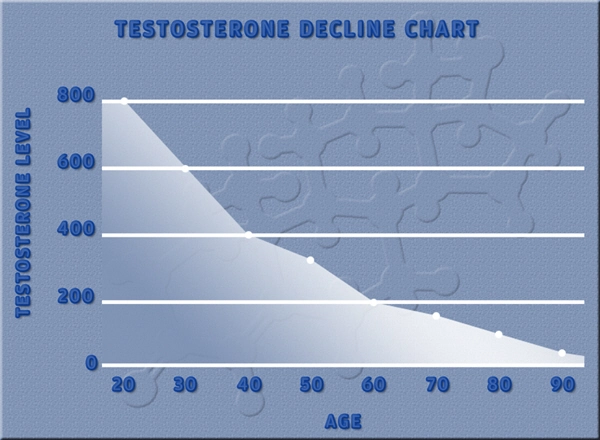
Testosterone replacement therapy (TRT) has become increasingly prevalent among American males seeking to mitigate the effects of hypogonadism and age-related testosterone decline. Among the various formulations available, testosterone undecanoate has gained popularity due to its long-acting nature and favorable pharmacokinetic profile. However, the long-term impact of this therapy on liver function remains a topic of significant interest and concern. This article aims to provide a retrospective analysis of the effects of long-term testosterone undecanoate use on liver function in American males, drawing upon recent research and clinical data.
Background on Testosterone Undecanoate
Testosterone undecanoate is an esterified form of testosterone designed for intramuscular injection, offering a sustained release of the hormone over several weeks. This formulation has been praised for its convenience and efficacy in maintaining stable serum testosterone levels. Despite its advantages, the potential hepatotoxicity associated with long-term use necessitates careful monitoring and evaluation.
Methodology of the Retrospective Analysis
Our analysis involved reviewing medical records and laboratory data from a cohort of American males who had been on testosterone undecanoate therapy for at least two years. Liver function tests, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT), were assessed at baseline and at regular intervals throughout the treatment period. Additionally, we considered potential confounding factors such as age, body mass index (BMI), and concurrent medication use.
Findings on Liver Function
The retrospective analysis revealed that the majority of participants maintained stable liver function parameters throughout the study period. Specifically, mean ALT and AST levels remained within the normal range, with only a small subset of individuals showing transient elevations. Notably, no significant trends towards progressive liver dysfunction were observed. These findings suggest that, for most American males, long-term use of testosterone undecanoate is not associated with clinically significant hepatotoxicity.
Impact of Dosage and Duration
Further analysis indicated that the dosage and duration of testosterone undecanoate therapy did not correlate strongly with changes in liver function tests. This observation supports the notion that, when used within recommended guidelines, testosterone undecanoate does not pose a substantial risk to liver health. However, it is crucial for healthcare providers to tailor dosages to individual patient needs and to monitor liver function regularly.
Consideration of Confounding Factors
Our study also examined the influence of confounding factors on liver function. Age and BMI were found to have a minimal impact on the observed liver function test results. However, concurrent use of medications known to affect liver function, such as statins or nonsteroidal anti-inflammatory drugs (NSAIDs), was associated with a higher likelihood of transient liver enzyme elevations. These findings underscore the importance of considering the patient's overall health and medication profile when assessing the safety of testosterone undecanoate therapy.
Clinical Implications and Recommendations
The results of this retrospective analysis provide reassurance regarding the long-term safety of testosterone undecanoate on liver function in American males. Nevertheless, clinicians should remain vigilant and conduct periodic liver function assessments, particularly in patients with pre-existing liver conditions or those taking hepatotoxic medications. Patient education on the importance of adherence to prescribed dosages and regular follow-up visits is also essential for optimizing the safety and efficacy of TRT.
Conclusion
In conclusion, our comprehensive review of long-term testosterone undecanoate use in American males indicates that this therapy is generally well-tolerated with respect to liver function. While the majority of patients experienced no significant liver enzyme elevations, ongoing monitoring remains crucial to ensure the continued safety of this treatment. As the use of TRT continues to rise, further research and vigilance will be necessary to fully understand its long-term effects on liver health and overall well-being.
Contact Us Today For A Free Consultation

- Testosterone Undecanoate: Enhancing Athletic Performance in American Males - Benefits and Risks [Last Updated On: March 5th, 2025] [Originally Added On: March 5th, 2025]
- Testosterone Undecanoate: Long-Acting Treatment for Hypogonadism in American Men [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Maximizing Testosterone Undecanoate Benefits: Diet, Exercise, and Lifestyle for American Men [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Testosterone Undecanoate: A Long-Acting TRT Option for American Males with Hypogonadism [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Testosterone Undecanoate: Enhancing Life Quality for American Males with Low Testosterone [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Testosterone Undecanoate: A Breakthrough in Treating Andropause for American Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Testosterone Undecanoate Therapy: Importance of Regular Monitoring for American Men [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Safety Profile of Testosterone Undecanoate in American Males: Monitoring and Management [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Testosterone Undecanoate: Managing Deficiency in Diverse American Male Demographics [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Testosterone Undecanoate Therapy Enhances Sleep Quality in American Men with Hypogonadism [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Testosterone Undecanoate's Impact on Hair Growth in American Males: Insights and Management [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Testosterone Undecanoate: A Solution for Muscle Loss in Aging American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Exploring Testosterone Undecanoate's Role in Managing Chronic Fatigue in Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Testosterone Undecanoate: Enhancing Fertility in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Testosterone Undecanoate: Cultural Perceptions and Healthcare Navigation in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Testosterone Undecanoate's Impact on Cognitive Function in American Men: A Review [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Testosterone Undecanoate: A Promising Solution for Weight Management in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Testosterone Undecanoate: Enhancing Emotional Well-being in American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Testosterone Undecanoate: A Promising Therapy for Hypogonadism in American Male Cancer Survivors [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Testosterone Undecanoate: Enhancing Injury Recovery in American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Testosterone Undecanoate: Enhancing Metabolic Health in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Testosterone Undecanoate: Effects on Blood Pressure in American Men with Hypogonadism [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Testosterone Undecanoate: A Vital Therapy for American Male Veterans' Health [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Testosterone Undecanoate: Efficacy and Safety in American Men - A Clinical Overview [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Testosterone Undecanoate: Managing Side Effects for American Men's Health [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Testosterone Undecanoate's Impact on Eye Health in American Men: Benefits and Risks [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Testosterone Undecanoate: A Promising Treatment for Sexual Dysfunction in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Testosterone Undecanoate's Impact on Immune Function in American Males: Benefits and Risks [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Testosterone Undecanoate: A Promising Treatment for Osteoporosis in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Testosterone Undecanoate's Impact on Respiratory Health in American Men: Benefits and Risks [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Testosterone Undecanoate: Enhancing Endurance in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Testosterone Undecanoate: Enhancing Skin Health in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Testosterone Undecanoate: Impacts on American Male Longevity and Health [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Testosterone Undecanoate's Impact on Dental Health in American Males: A Comprehensive Review [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Testosterone Undecanoate: Dispelling Myths and Understanding Benefits for Hypogonadism Treatment [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Testosterone Undecanoate's Impact on Digestive Health in American Males: Benefits and Risks [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Testosterone Undecanoate: Economic Impact and Healthcare Benefits for American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Testosterone Undecanoate: A Promising Stress Management Tool for American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Testosterone Undecanoate's Impact on Joint Health in American Males: Benefits and Risks [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Testosterone Undecanoate's Impact on Kidney Function in American Men: A Comprehensive Review [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Testosterone Undecanoate Enhances Skin Elasticity in American Men with Hypogonadism [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Testosterone Undecanoate's Impact on Liver Health in American Men: A Comprehensive Review [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Testosterone Undecanoate: Dosage Adjustments and Monitoring for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Testosterone Undecanoate: Enhancing Diabetes Management in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Testosterone Undecanoate: Optimizing Hypogonadism Treatment for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Testosterone Undecanoate's Impact on Cholesterol Levels in American Men: A Comprehensive Review [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Testosterone Undecanoate: Enhancing Cognitive Function in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Testosterone Undecanoate's Impact on Nail Health in American Males: Benefits and Risks [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Testosterone Undecanoate: A Novel Approach to Managing Allergies in American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Testosterone Undecanoate: A Promising Therapy for Chronic Pain in American Males [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Testosterone Undecanoate's Impact on Hearing in American Males: A Comprehensive Review [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Testosterone Undecanoate: Benefits and Considerations for American Men's Reproductive Health [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Testosterone Undecanoate: Enhancing Muscle, Reducing Fat in American Males [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Testosterone Undecanoate and Hair Loss: Insights for American Men on TRT [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Testosterone Undecanoate: Enhancing Immune Response in American Men [Last Updated On: April 4th, 2025] [Originally Added On: April 4th, 2025]
- Monitoring Testosterone Undecanoate Treatment: Key Parameters and Guidelines for American Men [Last Updated On: April 4th, 2025] [Originally Added On: April 4th, 2025]
- Testosterone Undecanoate's Impact on Blood Clotting in American Males: Risks and Management [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Testosterone Undecanoate: A Promising Treatment for Anxiety in American Males [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Testosterone Undecanoate: Enhancing Muscle Recovery and Performance in American Men [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Testosterone Undecanoate's Impact on Heart Rate in American Men: Safety and Efficacy [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Testosterone Undecanoate's Impact on Blood Sugar in American Males: Benefits and Risks [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Testosterone Undecanoate: A Promising Treatment for Depression in American Males [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Testosterone Undecanoate's Impact on Skin Pigmentation in American Males: Mechanisms and Clinical Insights [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Managing Side Effects of Testosterone Undecanoate Therapy in American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Testosterone Undecanoate: Enhancing Muscle Strength in American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Testosterone Undecanoate: Enhancing Male Sexual Health in American Men [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Testosterone Undecanoate: Enhancing Bone Healing in American Men [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Testosterone Undecanoate Enhances Wound Healing in American Men: Clinical Insights and Implications [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Testosterone Undecanoate's Impact on Appetite in American Males: A Comprehensive Analysis [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Testosterone Undecanoate's Impact on Thermoregulation in American Males: Benefits and Research Needs [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Testosterone Undecanoate's Impact on Sleep Quality in American Men: A Comprehensive Review [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Testosterone Undecanoate's Impact on Skin Sensitivity in American Males: A Comprehensive Review [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Testosterone Undecanoate Therapy: Lifestyle Adjustments for American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Testosterone Undecanoate Enhances Skin Hydration in American Males: A Comprehensive Study [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Testosterone Undecanoate: Enhancing Aesthetics in American Men Through Muscle and Fat Optimization [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Testosterone Undecanoate: A Promising Treatment for Migraines in American Males [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Testosterone Undecanoate's Impact on Vascular Health in American Men: A Comprehensive Review [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Testosterone Undecanoate's Impact on Blood Viscosity in American Men: Risks and Management [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Testosterone Undecanoate: Effective Long-Acting Treatment for Hypogonadism in American Men [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Testosterone Undecanoate: Benefits, Risks, and Management for Long-Term Use in American Men [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
Word Count: 596