Context: The risks of testosterone therapy in men remain poorly understood. Objective: The aim of this study was to conduct a systematic review and meta-analyses of testosterone trials to evaluate the adverse effects of testosterone ...
Context: The risks of testosterone therapy in men remain poorly understood. Objective: The aim of this study was to conduct a systematic review and meta-analyses of testosterone trials to evaluate the adverse effects of testosterone ...
Adverse Effects of Testosterone Therapy in Adult Men: A Systematic Review and Meta-Analysis
M. Mercè Fernández-Balsells, Mohammad Hassan Murad, Melanie Lane, Juliana F. Lampropulos, Felipe Albuquerque, Rebecca J. Mullan, Neera Agrwal, Mohamed B. Elamin, Juan F. Gallegos-Orozco, Amy T. Wang, Patricia J. Erwin, Shalender Bhasin, and Victor M. Montori
Address all correspondence and requests for reprints to: Victor M. Montori, M.D., M.Sc., Mayo Clinic, W18A, 200 First Street SW, Rochester, Minnesota 55905. E-mail: montori.victor@mayo.edu.
DOI: dx.doi.org/10.1210/jc.2009-2575
Received: December 03, 2009
Accepted: March 19, 2010
Published Online: July 02, 2013
Abstract
Context: The risks of testosterone therapy in men remain poorly understood.
Objective: The aim of this study was to conduct a systematic review and meta-analyses of testosterone trials to evaluate the adverse effects of testosterone treatment in men.
Data Sources: We searched MEDLINE, EMBASE, and Cochrane CENTRAL from 2003 through August 2008. Review of reference lists and contact with experts further identified candidate studies.
Study Selection: Eligible studies were comparative, randomized, and nonrandomized and reported the effects of testosterone on outcomes of interest (death, cardiovascular events and risk factors, prostate outcomes, and erythrocytosis). Reviewers, working independently and in duplicate, determined study eligibility.
Data Extraction: Reviewers working independently and in duplicate determined the methodological quality of studies and collected descriptive, quality, and outcome data.
Data Synthesis: The methodological quality of the 51 included studies varied from low to medium, and follow-up duration ranged from 3 months to 3 yr. Testosterone treatment was associated with a significant increase in hemoglobin [weighted mean difference (WMD), 0.80 g/dl; 95% confidence interval (CI), 0.45 to 1.14] and hematocrit (WMD, 3.18%; 95% CI, 1.35 to 5.01), and a decrease in high-density lipoprotein cholesterol (WMD, ?0.49 mg/dl; 95% CI, ?0.85 to ?0.13). There was no significant effect on mortality, prostate, or cardiovascular outcomes.
Conclusions: The adverse effects of testosterone therapy include an increase in hemoglobin and hematocrit and a small decrease in high-density lipoprotein cholesterol. These findings are of unknown clinical significance. Current evidence about the safety of testosterone treatment in men in terms of patient-important outcomes is of low quality and is hampered by the brief study follow-up.
Current evidence suggests that testosterone use in men causes erythrocytosis, but evidence about cardiovascular events and prostate outcomes is limited and imprecise.
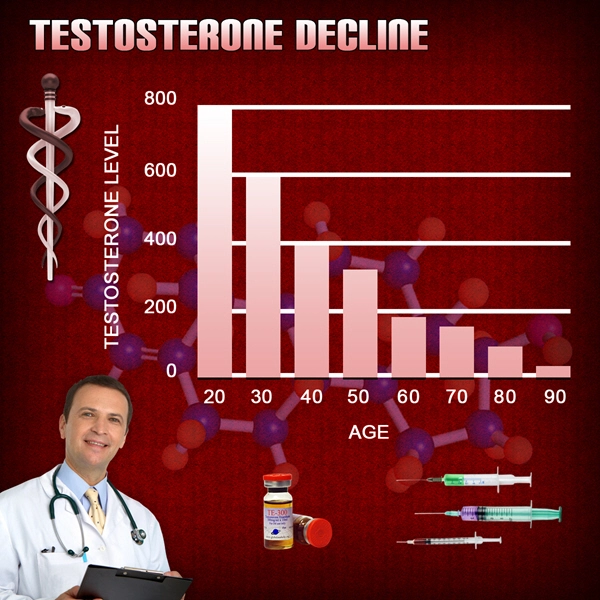
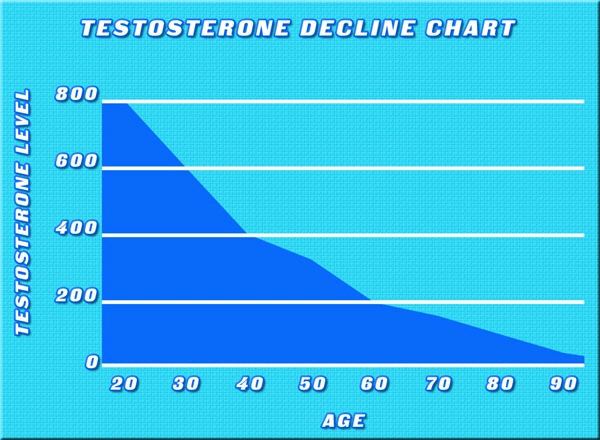
Although the prevalence of low total testosterone levels in men increases steadily from 20 to 50% from the sixth to ninth decades of life (1), the utility of testosterone therapy in aging men remains controversial. Meta-analyses report small improvements in domains of sexual function, bone mineral density, muscle mass, and grip strength (2, 3, 4, 5). However, the risks of testosterone therapy remain poorly understood. The Institute of Medicine Expert Panel suggested that safety trials of testosterone replacement should be deferred until the efficacy of this treatment has been demonstrated. Therefore, adequately powered randomized trials to ascertain the effects of testosterone therapy on prostate and cardiovascular health are unlikely to be conducted any time soon. Studies designed to address safety are lacking, and the evidence of harm is mainly based on the reported adverse events in trials designed to address the efficacy of this treatment. Most of these trials included relatively small samples, and each study individually had only a small number of adverse events. Therefore, this investigation aimed to conduct a systematic review of the literature and perform meta-analyses of the adverse effects of testosterone therapy.
In 2005, a meta-analysis of the adverse effects of testosterone replacement therapy (6) showed that elevated hematocrit and an increase in a composite of prostate-related events were the most common side effects of testosterone replacement therapy. Imprecision due to the small number of events hampered inferences regarding differences in the rates of prostate cancer, cardiovascular events, sleep apnea, or death. In 2007, another meta-analysis failed to show differences in blood pressure, glycemia, and lipid fractions, but it reported a trend for increased cardiovascular events associated with testosterone use [pooled odds ratio, 1.82; 95% confidence interval (CI), 0.78–4.23] (7). Overall, the studies included in both meta-analyses were randomized controlled trials (RCTs) that enrolled a relatively small number of patients and had short follow-up and high loss to follow-up rates.
Since the publication of these meta-analyses, several larger studies of the effects of testosterone therapy on muscle performance and physical function, glucose metabolism (8, 9, 10, 11), prostate outcomes (10), and the performance status of patients with heart failure (12) have been published. Because of these and other published trials, the Endocrine Society Task Force on Testosterone Use in Adult Men requested an updated systematic review of randomized trials and observational studies with long-term follow-up to determine the possible adverse effects of testosterone therapy, including cardiovascular and prostate outcomes.
Materials and Methods
We developed a systematic review protocol with input from the expert members of the commissioning Task Force from The Endocrine society. This report adheres to the reporting guidelines of systematic reviews (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (13).
Eligibility criteria
Eligible studies were comparative studies (randomized and nonrandomized) that enrolled adult men with low or low-normal testosterone levels and treated with any testosterone formulation for at least 3 months. The studies had to have a control group without testosterone use and measure the outcomes of interest.
The outcomes of this systematic review are:
Prostate outcomes: the diagnosis of prostate cancer, prostate-specific antigen (PSA) levels above 4 ng/ml or a significant increase in PSA during treatment (defined as PSA increment of >1.4 ng/ml above baseline), a prostatic biopsy, increase in International Prostate Symptom Score (IPSS) greater than 4, acute urinary retention, and a composite prostate endpoint that combines these aforementioned outcomes.
Cardiovascular events and cardiometabolic risk factors: death, coronary events (fatal and nonfatal myocardial infarction, hospitalization for an acute coronary syndrome, coronary revascularization), cerebrovascular events (fatal and nonfatal stroke or transient ischemic attack), peripheral vascular events (new onset claudication, acute arterial occlusion, revascularization procedure), changes in lipid fractions, changes in glucose metabolism (fasting glucose level and new onset of diabetes), and blood pressure.
Indices of red cell mass (hemoglobin, hematocrit, and incidence of erythrocytosis defined as hematocrit >52%).
We excluded studies that used androgens other than testosterone as well as studies with simultaneous treatment with other hormones and drugs unless there was a clearly defined treatment arm that received only testosterone treatment. Studies not reporting the outcomes of interest were excluded. Language of publication was not an eligibility criterion.
Study identification
An expert reference librarian (P.J.E.) conducted the electronic search with input from study investigators with expertise in systematic reviews (V.M.M. and M.H.M.). We searched MEDLINE, EMBASE, and Cochrane CENTRAL electronic databases from 2003 through August 2008. Studies published before 2003 were obtained from two published systematic reviews (6, 7). Because published reviews excluded patients with HIV/AIDS, the previous search strategy was expanded to cover the period 1981–2004, focusing on studies conducted in this population [exp HIV infections/ or (human adj immune*) or (acquired adj immune*) or AIDS* or HIV]. The detailed strategy is available upon request. To identify additional candidate studies, we reviewed the reference lists of the eligible primary studies, narrative reviews, and systematic reviews, and we queried the expert members of the commissioning task force.
Study selection
Working independently and in duplicate, reviewers screened all abstracts and titles. After obtaining all potentially eligible studies in full text, these reviewers, again working independently and in duplicate, determined eligibility with acceptable chance-adjusted agreement (? = 0.85; range, 0.75–0.94). Disagreements were resolved by consensus or arbitration.
Data collection
Using a standardized data extraction form and working in duplicate, we abstracted the following descriptive data from each study: description of study participants (age, baseline testosterone levels), and characteristics of treatment and control interventions (testosterone formulation, dose, frequency, route of administration, and treatment duration). We extracted the outcomes of interest at the longest point of complete follow-up. We contacted the authors of included studies by e-mail if a clarification of their methods (e.g. randomization and blinding procedures) or results (missing data, e.g. sd) was needed.
Quality assessment
We employed the GRADE approach to rate the quality of evidence (14). To assess the methodological quality of randomized trials, we determined how the randomization sequence was generated, how allocation was concealed, whether there were important imbalances at baseline, which groups were blinded (patients, caregivers, data collectors, outcome assessors, data analysts), what the loss to follow-up rate was (in the intervention and the control arm), whether the analyses were by intention to treat, and how missing outcome data were dealt with. For each study, we also assessed how the population was selected, the duration and route of testosterone administration, the adequacy of study follow-up, and the funding source. We assessed our chance-adjusted agreement on study quality using the ? statistic with disagreements resolved by consensus or arbitration.
Meta-analyses
We estimated the relative risk (RR) for dichotomous outcomes and the weighted mean difference (WMD) for continuous outcomes pooled across studies using the DerSimonian and Laird random-effects model (15). We quantified inconsistency using the I2 statistic, which describes the proportion of heterogeneity across studies that is not due to chance, thus describing the extent of true inconsistency in results across trials (16). I2 less than 25% and I2 more than 50% reflect small and significant inconsistency, respectively.
Subgroup and sensitivity analyses
To explore causes of inconsistency and subgroup-treatment interactions, subgroup analyses were specified a priori according to the following factors:
Participants: age less than 65 or more than 65 yr; total testosterone level at baseline (low if less than 300 ng/dl or 10.4 nmol/l). If total testosterone was not available, then lower limit of normal for bioavailable or free testosterone levels. If neither total nor free testosterone levels were available, then studies were classified according to participant characteristics.
Interventions: testosterone formulation, route of administration, and dose (substitution doses as recommended by The Endocrine Society vs. anything higher).
Outcome characteristic: duration of follow-up (less than 6 months vs. more than 6 months).
Study quality measure: proportion of patients lost to follow-up (10% or less vs. more than 10%), concealment of allocation, blinding of patients, health care professionals, data collectors, and outcome assessors.
In addition, we conducted sensitivity analyses using Peto odds ratio and different continuity correction factors to determine whether these choices in analysis methods affected the conclusions.
Results
Search results
The search identified 984 candidate references, of which 51 trials were deemed eligible (Fig. 1). The characteristics of included studies are summarized in Table 1. Studies that did not provide sufficient data for this meta-analysis are described in Table 2.
Methodological quality
The overall quality of the included studies varied from low to medium (Table 3). Several studies did not provide explicit description of methods to protect against bias, whereas in other studies, it was clear that such techniques were not used. Only a few studies reported allocation concealment and blinding of the outcome assessors. Loss to follow-up was, in most cases, substantial, with 10 studies losing more than 20% of participants, and differed between study arms in five studies (17, 18, 19, 20, 21).
Mortality and cardiovascular outcomes
There were no significant differences in the rates of death, myocardial infarction, revascularization procedures, or cardiac arrhythmias between the testosterone and the placebo/nonintervention groups (Fig. 2). Heterogeneity was low or incalculable due to the small number of studies in these analyses.
Cardiovascular risk factors
The testosterone and placebo/nonintervention groups did not differ significantly in the incidence of diabetes mellitus or in the changes from baseline in cardiometabolic risk factors, such as fasting glucose, total and low-density lipoprotein (LDL) cholesterol, triglycerides, systolic and diastolic blood pressure levels. High-density lipoprotein (HDL) cholesterol levels were significantly lower in the testosterone-treated group than the control group (WMD, ?0.49 mg/dl; 95% CI, ?0.85 to ?0.13; I2 = 69%) (Table 4). The majority of these analyses were associated with significant heterogeneity.
Prostatic/urological outcomes
There was no significant effect of testosterone therapy on patient-important outcomes such as the incidence of prostatic cancer or the need for prostate biopsy, when compared with the placebo/nonintervention group (Fig. 2). There was no significant difference between the two groups in the risk of other prostatic and urological outcomes such as a significant increase of PSA, changes in IPSS lower urinary tract symptoms, or the composite prostate outcome (Table 4).
Erythrocytosis
Hemoglobin (WMD, 0.80 g/dl; 95% CI, 0.45 to 1.14; I2 = 95%) and hematocrit (WMD, 3.18%; 95% CI, 1.35 to 5.01; I2 = 91%) levels increased to a greater extent in testosterone-treated men than in men receiving placebo or no treatment. Testosterone-treated men were at higher risk of developing erythrocytosis than the placebo/nonintervention group (RR, 3.15; 95% CI, 1.56 to 6.35; I2 = 0%) (Table 4).
Subgroup analysis and sensitivity analysis
We performed subgroup analyses to explore possible causes of heterogeneity and to detect subgroup-treatment interactions. These analyses are presented in the table in the Appendix (published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). The route of testosterone administration and dose partially explained the heterogeneity of results. Intramuscular route and high dose were associated with greater increases in red cell indices (hemoglobin and hematocrit), PSA, and systolic and diastolic blood pressure. Higher doses were associated also with more increase in LDL and total cholesterol. Baseline testosterone status affected the response of hemoglobin and hematocrit; patients with low baseline had more increase of both indices. The population characteristics (healthy vs. having comorbid conditions such as end stage renal disease, HIV, congestive heart failure, and coronary artery disease) affected the effect size for several outcomes, suggesting an obvious source of heterogeneity; however, these effects were paradoxical and difficult to interpret in a summary meta-analysis of a study-level aggregate data, as is the case in this analysis. We used Peto odds ratio and the treatment arm method for continuity correction in sensitivity analyses, which are the recommended approaches to deal with studies with low event rates (sparse data); the use of these methods did not change study conclusions. The exclusion of studies in which the loss of follow-up significantly differed between the intervention and control groups did not change the conclusions of this report.
Discussion
Principal findings
This systematic review and meta-analyses demonstrated that testosterone therapy in men was associated with significant increases in hemoglobin and hematocrit. There also was a statistically significant but small reduction in HDL cholesterol. However, testosterone therapy had no significant effects on all-cause mortality, prostatic or urological outcomes, cardiovascular events, or cardiovascular risk factors. Several subgroup interactions were found and can partially explain the heterogeneity associated with the analysis. However, caution should be exercised in interpreting these analyses because they are considered observational in nature (despite the fact that the original studies were randomized) and the associations found can be attributable to chance due to the multiple simultaneous comparisons. Subgroup interactions generated by study-level meta-analyses are considered hypothesis-generating and should be confirmed at a patient-level (in a large trial or individual patient meta-analysis) before clinical implications are inferred.
Limitations and strengths
The strengths of this study include the comprehensive literature search, the application of bias protection measures in the selection of the studies, and the evaluation of their methodological quality. Nevertheless, the quality of the evidence varied from low to medium considering the imprecision (small number of events), heterogeneity (for the outcomes of cardiometabolic risk factors, hemoglobin and hematocrit), and methodological limitations of the included trials. In particular, the brief duration of most testosterone trials limited inferences about the long-term safety of this treatment. In addition, publication and reporting biases likely affected the inferences in this review because not all studies reported the outcomes of interest (22).
Implications for practice and research
Because increases in hemoglobin and hematocrit are the most frequent adverse events associated with testosterone therapy, these data support the Endocrine Society Clinical Practice Guideline that hemoglobin and hematocrit should be monitored in androgen-deficient men receiving testosterone therapy (23). Studies of longer treatment duration that assess patient-important outcomes are needed. Despite the high prevalence of low testosterone levels in adult men, the published literature is not very helpful in addressing the safety concerns of patients and clinicians attempting to weigh the long-term potential benefits and harms of testosterone treatment.
Comparison with previous reviews
Our review added several new studies of substantially larger sample size to prior systematic reviews and reflects the current state of the available evidence. Our results are consistent with previous reviews (6, 7) to a great extent in terms of the inability to find a statistically significant effect of testosterone treatment on patient-important adverse outcomes, such as death, cardiovascular events, the incidence of prostate cancer, or worsening of lower urinary tract symptoms. Despite the increase in the number of studies and sample size since previous reviews, these risk estimates for these outcomes continue to be imprecise.
Testosterone therapy for age-related decline in testosterone therapy in middle-aged and older men remains a controversial issue. Although there is agreement that total and free testosterone levels decline with advancing age, neither the benefits of testosterone treatment nor its safety have been established. Although testosterone therapy has been shown to increase lean body mass, muscle strength, vertebral bone mineral density, and some domains of sexual function, the benefits of therapy on patient-important outcomes–disability, vitality, fractures, and quality of life–remain largely unknown (1, 18). Our search of published comparative observational studies, which often provide good evidence for harm questions due to longer follow-up and larger sample size, did not render significant contribution to the existing knowledge. Future studies, both randomized and observational, are needed and should have longer follow-up. For example, the Testosterone Trials (ClinicalTrials.gov:NCT00799617), a multicenter coordinated set of trials involving 12 clinical sites across the United States, will randomly assign elderly men whose serum testosterone concentrations are unequivocally low to either placebo or testosterone therapy for 12 months. This landmark trial will advance our knowledge about testosterone effects on physical and sexual function, vitality, cognition, and anemia. However, this trial is not adequately powered to determine the long-term harms associated with treatment. Therefore, periodic collation of adverse event data across trials through the use of systematic reviews and meta-analyses will be helpful.
Conclusions
Testosterone treatment in adult men is associated with an increase in hemoglobin and hematocrit and a small reduction in HDL cholesterol. The clinical significance of these findings and the effects on patient-important outcomes such as mortality, cardiovascular events, and incidence of prostate cancer requires further investigation.
This work was supported by a contract from The Endocrine Society. M.M.F.-B. has received grant support from the Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo (BA08/90035), Government of Spain.
Disclosure Summary: M.M.F.-B., M.H.M., M.L., J.F.L., F.A., R.J.M., N.A., M.B.E., J.F.G.-O., A.T.W., P.J.E., S.B., and V.M.M. have nothing to declare.
Abbreviations: CI, Confidence interval; HDL, high-density lipoprotein; IPSS, International Prostate Symptom Score; LDL, low-density lipoprotein; PSA, prostate-specific antigen; RCT, randomized controlled trial; RR, relative risk; WMD, weighted mean difference.
Read more here:
Adverse Effects of Testosterone Therapy in Adult Men: A Systematic ...
Contact Us Today For A Free Consultation

- Low Testosterone Levels, Foods That Increase Testosterone Levels wwwSelf-Improvement-Bible.com [Last Updated On: November 12th, 2023] [Originally Added On: May 30th, 2011]
- Low Testosterone in Men: The Next Big Thing in Medicine! - Abraham Morgentaler, MD [Last Updated On: May 7th, 2023] [Originally Added On: June 3rd, 2011]
- How To Determine Testosterone Levels By Looking At Your Ring Finger [Last Updated On: December 7th, 2017] [Originally Added On: June 30th, 2011]
- Prolab Horny Goat Weed Testosterone Booster Supplement Review [Last Updated On: November 23rd, 2023] [Originally Added On: July 19th, 2011]
- The Healthy Skeptic: Products make testosterone claims [Last Updated On: August 13th, 2024] [Originally Added On: September 11th, 2011]
- How To Naturally Increase Testosterone [Last Updated On: November 21st, 2023] [Originally Added On: September 28th, 2011]
- Testosterone Production - Video [Last Updated On: November 25th, 2024] [Originally Added On: November 20th, 2011]
- Testosterone makes us less cooperative and more egocentric, study finds [Last Updated On: January 23rd, 2018] [Originally Added On: February 1st, 2012]
- Testosterone makes us less cooperative and more egocentric [Last Updated On: January 24th, 2018] [Originally Added On: February 1st, 2012]
- Too much testosterone makes for bad decisions, tests show [Last Updated On: April 30th, 2025] [Originally Added On: February 1st, 2012]
- Today in Research: Testosterone's Negative Effects; Diet Soda Death [Last Updated On: January 2nd, 2018] [Originally Added On: February 2nd, 2012]
- Testosterone drives ego, trips cooperation [Last Updated On: December 2nd, 2017] [Originally Added On: February 4th, 2012]
- FDA approves BioSante/Teva's testosterone gel [Last Updated On: April 28th, 2025] [Originally Added On: February 15th, 2012]
- 'Manly' Fingers Make For Strong Jawline in Young Boys [Last Updated On: December 1st, 2017] [Originally Added On: February 15th, 2012]
- Teva, BioSante Win U.S. Approval for Testosterone Therapy [Last Updated On: December 10th, 2017] [Originally Added On: February 15th, 2012]
- BioSante Gains on Approval of Testosterone Gel: Chicago Mover [Last Updated On: January 8th, 2018] [Originally Added On: February 16th, 2012]
- BioSante soars following drug approval from FDA [Last Updated On: December 26th, 2017] [Originally Added On: February 16th, 2012]
- Antibodies, Not Hard Bodies: The Real Reason Women Drool Over Brad Pitt [Last Updated On: December 24th, 2017] [Originally Added On: February 21st, 2012]
- Almark Publishing Releases Book From Mark Rosenberg, M.D. Revealing Natural Discoveries Associated With Low ... [Last Updated On: May 3rd, 2025] [Originally Added On: February 28th, 2012]
- Testosterone Replacement Clinic Comes to Kansas City with Potential to Help Thousands of Men [Last Updated On: May 2nd, 2025] [Originally Added On: March 1st, 2012]
- Study examines the relative roles of testosterone and its metabolite, dihydrotestosterone in men [Last Updated On: December 2nd, 2017] [Originally Added On: March 7th, 2012]
- The Role of 5{alpha}-Reductase Inhibition in Men Receiving Testosterone Replacement Therapy [Editorial] [Last Updated On: December 21st, 2017] [Originally Added On: March 7th, 2012]
- Effect of Testosterone Supplementation With and Without a Dual 5{alpha}-Reductase Inhibitor on Fat-Free Mass in Men ... [Last Updated On: January 3rd, 2018] [Originally Added On: March 7th, 2012]
- Why We Like Men Who Can Keep Their Cool [Last Updated On: December 30th, 2017] [Originally Added On: March 7th, 2012]
- Testosterone And Heart Health [Last Updated On: May 1st, 2025] [Originally Added On: March 10th, 2012]
- Your Life on Testosterone: Overly Sure of Yourself, Unwilling to Listen [Last Updated On: November 25th, 2018] [Originally Added On: March 15th, 2012]
- Mayo Clinic-TGen study role testosterone may play in triple negative breast cancer [Last Updated On: December 8th, 2017] [Originally Added On: March 23rd, 2012]
- A dose of testosterone might not cure what ails you [Last Updated On: January 23rd, 2018] [Originally Added On: March 25th, 2012]
- Green tea could aid athletes hide testosterone doping [Last Updated On: December 16th, 2017] [Originally Added On: March 25th, 2012]
- TGen Study Role Testosterone May Play in Triple Negative Breast Cancer [Last Updated On: December 6th, 2017] [Originally Added On: March 26th, 2012]
- Testosterone low, but responsive to competition, in Amazonian tribe [Last Updated On: January 23rd, 2018] [Originally Added On: March 28th, 2012]
- Competition-linked bursts of testosterone are fundamental aspect of human biology, study of Amazonian tribe suggests [Last Updated On: December 25th, 2017] [Originally Added On: March 28th, 2012]
- Playing football boosts testosterone levels by 30 percent! [Last Updated On: February 4th, 2024] [Originally Added On: March 28th, 2012]
- Testosterone low, but responsive to competition, in Amazonian tribe -- with slideshow [Last Updated On: December 9th, 2017] [Originally Added On: March 28th, 2012]
- The benefits of testosterone pellet therapy [Last Updated On: January 24th, 2018] [Originally Added On: March 29th, 2012]
- Low testosterone levels cause health woes [Last Updated On: November 25th, 2018] [Originally Added On: March 30th, 2012]
- Heart Failure Patients Getting Relief from Testosterone Supplements [Last Updated On: May 5th, 2025] [Originally Added On: April 21st, 2012]
- Study Finds Fatherhood Suppresses Testosterone [Last Updated On: May 4th, 2025] [Originally Added On: May 3rd, 2012]
- Low testosterone levels could raise diabetes risk for men [Last Updated On: January 26th, 2018] [Originally Added On: May 5th, 2012]
- Why low testosterone may increase your risk of diabetes [Last Updated On: November 25th, 2024] [Originally Added On: May 5th, 2012]
- Diabetes link to low testosterone [Last Updated On: November 25th, 2024] [Originally Added On: May 5th, 2012]
- Testosterone Linked to Weight Loss in Obese Men [Last Updated On: January 2nd, 2018] [Originally Added On: May 11th, 2012]
- Testosterone may help weight loss [Last Updated On: November 25th, 2024] [Originally Added On: May 11th, 2012]
- Testosterone-fuelled infantile males might be a product of Mom's behaviour [Last Updated On: December 25th, 2017] [Originally Added On: May 11th, 2012]
- Testosterone-fueled infantile males might be a product of Mom's behavior [Last Updated On: January 6th, 2018] [Originally Added On: May 11th, 2012]
- Testosterone supplements may help obese men lose weight [Last Updated On: January 5th, 2018] [Originally Added On: May 11th, 2012]
- Testosterone supplements 'can help men lose their middle-aged spread' [Last Updated On: November 25th, 2024] [Originally Added On: May 12th, 2012]
- Some doctors question safety of testosterone replacement therapy [Last Updated On: January 20th, 2018] [Originally Added On: May 15th, 2012]
- Health Canada Approves New Testosterone Topical Solution for Men [Last Updated On: May 15th, 2025] [Originally Added On: May 15th, 2012]
- Environment trumps genes in testosterone levels, study finds [Last Updated On: May 8th, 2025] [Originally Added On: May 15th, 2012]
- Global Testosterone Replacement Therapy (TRT) Industry [Last Updated On: May 7th, 2025] [Originally Added On: May 21st, 2012]
- Testosterone Fuels Boom, Swindler Sows Panic: Top Business Books [Last Updated On: January 13th, 2018] [Originally Added On: June 2nd, 2012]
- Increase in testosterone drug use [Last Updated On: April 12th, 2018] [Originally Added On: June 4th, 2012]
- Testosterone Promotes Agression Automatically [Last Updated On: January 29th, 2018] [Originally Added On: June 9th, 2012]
- Testosterone shown to help sexually frustrated women [Last Updated On: January 27th, 2018] [Originally Added On: June 9th, 2012]
- Research and Markets: Testosterone Replacement Therapy (TRT) - Global Strategic Business Report [Last Updated On: December 23rd, 2017] [Originally Added On: June 12th, 2012]
- Proposed testosterone testing of some female olympians challenged by Stanford scientists [Last Updated On: January 30th, 2018] [Originally Added On: June 14th, 2012]
- Testosterone Makes Bosses Into Jerks, Says Paul Zak [Last Updated On: January 8th, 2018] [Originally Added On: June 14th, 2012]
- Testosterone Therapy: A Misguided Approach to Erectile Dysfunction (ED) [Last Updated On: May 10th, 2025] [Originally Added On: June 20th, 2012]
- New drugs, new ways to target androgens in prostate cancer therapy [Last Updated On: January 8th, 2018] [Originally Added On: June 20th, 2012]
- Long-term testosterone treatment for men results in reduced weight and waist size [Last Updated On: January 19th, 2018] [Originally Added On: June 23rd, 2012]
- Declining testosterone levels in men not part of normal aging, study finds [Last Updated On: December 27th, 2017] [Originally Added On: June 23rd, 2012]
- Low testosterone not normal part of aging [Last Updated On: December 22nd, 2017] [Originally Added On: June 25th, 2012]
- Testosterone Does Not Necessarily Wane With Age [Last Updated On: December 6th, 2017] [Originally Added On: June 25th, 2012]
- Overweight men can boost low testosterone levels by losing weight [Last Updated On: December 10th, 2017] [Originally Added On: June 25th, 2012]
- Testosterone-replacement therapy improves symptoms of metabolic syndrome [Last Updated On: January 14th, 2018] [Originally Added On: June 26th, 2012]
- Testosterone therapy takes off pounds [Last Updated On: December 11th, 2017] [Originally Added On: June 26th, 2012]
- Weight loss may boost men's testosterone [Last Updated On: May 9th, 2025] [Originally Added On: June 27th, 2012]
- Low Testosterone? Study finds age may not be to blame [Last Updated On: May 12th, 2025] [Originally Added On: July 1st, 2012]
- Do you have low testosterone? [Last Updated On: December 15th, 2017] [Originally Added On: July 8th, 2012]
- Wall Streeters Buying Testosterone for an Edge [Last Updated On: May 11th, 2025] [Originally Added On: July 12th, 2012]
- Beefy Wall Street Traders rub on testosterone [Last Updated On: February 20th, 2024] [Originally Added On: July 12th, 2012]
- Tale of two runners exposes flawed Olympic thinking [Last Updated On: December 23rd, 2024] [Originally Added On: July 19th, 2012]
- Genetic markers for testosterone and estrogen level regulation identified [Last Updated On: January 6th, 2018] [Originally Added On: July 20th, 2012]
- BUSM researchers identify genetic markers for testosterone, estrogen level regulation [Last Updated On: December 18th, 2017] [Originally Added On: July 20th, 2012]
- DRS. OZ AND ROIZEN: How to reap the benefits of normal testosterone levels [Last Updated On: December 23rd, 2024] [Originally Added On: July 21st, 2012]
- How Testosterone Drives History [Last Updated On: December 24th, 2024] [Originally Added On: July 22nd, 2012]
- Testosterone replacement is "fountain of youth" for men [Last Updated On: January 3rd, 2018] [Originally Added On: July 27th, 2012]
- Pill for low testosterone in men heads for phase II clinical trials [Last Updated On: December 31st, 2017] [Originally Added On: August 2nd, 2012]
- Treating low testosterone [Last Updated On: December 11th, 2017] [Originally Added On: August 5th, 2012]
Word Count: 3124