By RTT News, February 25, 2015, 07:17:00 AM EDT
(RTTNews.com) - Antares Pharma Inc. ( ATRS ) announced positive top-line pharmacokinetic results that showed that the primary endpoint was achieved in the Company's ongoing, multi-center, phase 3 clinical study or QST-13-003 evaluating the efficacy and safety of testosterone enanthate administered once-weekly by subcutaneous injection using the QuickShot auto injector in testosterone deficient adult males.
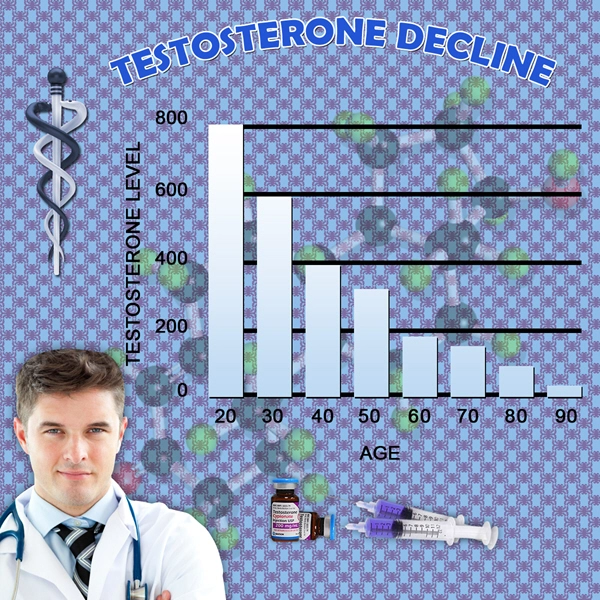
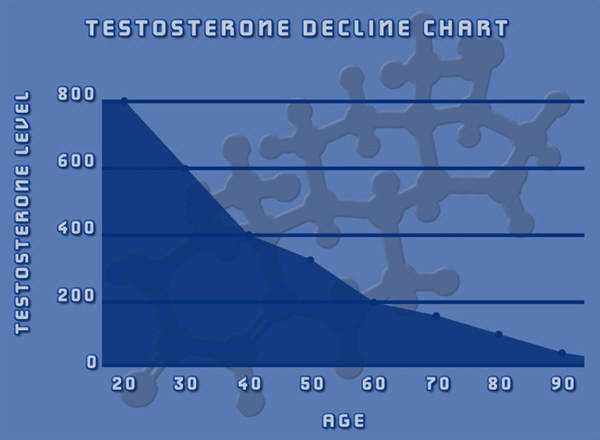
In the study, 150 adult males with hypogonadism (low testosterone) and testosterone blood levels less than 300 ng/dL received a starting dose of 75 mg of subcutaneously administered testosterone enanthate (QuickShot Testosterone, or QS T) once weekly for six weeks. Blinded adjustments to dose were made when necessary at week seven based upon the week six pre-dose blood level, and full pharmacokinetic (PK) profiles were obtained during the 12th week of treatment.
The protocol for the study required that at the week 12 endpoint: at least 75% of all patients' Cavg are within the normal range of 300 to 1100 ng/dL, with a lower limit of a 95% 2-sided confidence interval of greater than or equal to 65%; at least 85% of patients' Cmax are less than 1500 ng/dL; and no more than 5% of patients had a Cmax greater than 1800 ng/dL.
The primary endpoint of the population that received one or more doses of QS T was met by 139 out of 150 patients, equating to 92.7% with a 95% confidence interval of 87.3% to 96.3%. Among the 137 patients that completed all 12 weeks of dosing and PK sampling, 98.5% were within the pre-defined range.
The company noted that Participants in the study will remain on QS T and will be followed for an additional 40 weeks, and the collection of safety data is ongoing.
One hundred fifty patients have received at least one dose of study drug and to date, there have been no reported deaths and one serious adverse event (SAE) of hospitalization for worsening depression. This patient received a single dose of QS T, and the SAE was not considered to be related to study drug. Thus far, there have been no reported adverse events consistent with urticaria (hives), the company said.
For comments and feedback: contact editorial@rttnews.com
See the original post:
Antares Announces Positive Pharmacokinetic Results From QuickShot Phase 3 Study
Contact Us Today For A Free Consultation

- Low Testosterone Treatments - Video [Last Updated On: December 21st, 2024] [Originally Added On: July 12th, 2012]
- Treating low testosterone - Video [Last Updated On: December 21st, 2024] [Originally Added On: July 12th, 2012]
- Fatigue and Low Testosterone part 2 - Video [Last Updated On: December 22nd, 2024] [Originally Added On: July 12th, 2012]
- Fatigue and Low Testosterone part 1 - Video [Last Updated On: December 22nd, 2024] [Originally Added On: July 12th, 2012]
- Nation + World [Last Updated On: January 22nd, 2018] [Originally Added On: September 10th, 2012]
- Natural Remedies - Natural Way to Promote Healthy Vitality in Men. [Last Updated On: May 19th, 2025] [Originally Added On: October 6th, 2012]
- ‘Horrendous’ Study Shows Obese Teen Boys Face Impotence and Infertility [Last Updated On: January 11th, 2018] [Originally Added On: October 17th, 2012]
- Symptoms of Low Testosterone Levels in Men - Video [Last Updated On: January 6th, 2025] [Originally Added On: November 2nd, 2012]
- Symptoms of Low Testosterone Levels with Dr. Alexander - Video [Last Updated On: January 6th, 2025] [Originally Added On: November 2nd, 2012]
- Bio-Identical Hormone Therapy [Last Updated On: January 7th, 2025] [Originally Added On: November 2nd, 2012]
- Low Testosterone and Male Fertility - Video [Last Updated On: January 7th, 2025] [Originally Added On: November 2nd, 2012]
- He's the one they call Dr Feel Good! - Video [Last Updated On: January 8th, 2025] [Originally Added On: November 2nd, 2012]
- Get the down low on Low T - Video [Last Updated On: January 8th, 2025] [Originally Added On: November 2nd, 2012]
- Low Testosterone Quiz - Video [Last Updated On: January 9th, 2025] [Originally Added On: November 2nd, 2012]
- Female sex-enhancing nasal spray undergoing clinical trials [Last Updated On: October 24th, 2015] [Originally Added On: November 2nd, 2012]
- More Men Suffering from Low Testosterone [Last Updated On: May 28th, 2025] [Originally Added On: November 7th, 2012]
- obese men lose weight with testosterone - Video [Last Updated On: January 23rd, 2025] [Originally Added On: November 7th, 2012]
- Bodybuilding Tip Testosterone Boosters to Build Muscle Do They Work - Video [Last Updated On: January 24th, 2025] [Originally Added On: November 9th, 2012]
- Male Pattern Baldness - Video [Last Updated On: January 27th, 2025] [Originally Added On: November 12th, 2012]
- Bio-Identical Hormone Replacement Men and/or Women - Video [Last Updated On: February 3rd, 2025] [Originally Added On: November 22nd, 2012]
- LEARNING ABOUT TESTOSTERONE LEVELS - Video [Last Updated On: February 4th, 2025] [Originally Added On: November 22nd, 2012]
- Salivary diagnostics [Last Updated On: February 11th, 2025] [Originally Added On: December 4th, 2012]
- Natural Hormone Repalcement Therapy [Last Updated On: February 19th, 2025] [Originally Added On: December 10th, 2012]
- Why You Should Help Others Fight -Major Depression, Anxiety, Depersonalization, Low Testosterone- - Video [Last Updated On: January 7th, 2013] [Originally Added On: January 7th, 2013]
- Effects Of Low Testosterone | Low T Hormones - Video [Last Updated On: February 14th, 2013] [Originally Added On: February 14th, 2013]
- Go7Herbal Why Men Are Having Low Testosterone And High Estrogen - Video [Last Updated On: February 18th, 2013] [Originally Added On: February 18th, 2013]
- Vegan Blood Test - I'm Diabetic, Obese, and Low Testosterone - Video [Last Updated On: February 23rd, 2013] [Originally Added On: February 23rd, 2013]
- Vegan for 12 years. Diabetic, Obese, Low Testosterone, Hyperkalemic and out of shape? - Video [Last Updated On: February 26th, 2013] [Originally Added On: February 26th, 2013]
- Low testosterone in men-Have your testosterone checked for free. See below for details. - Video [Last Updated On: March 7th, 2013] [Originally Added On: March 7th, 2013]
- Low Testosterone (Lunch with the Doctor) February 2013 - Video [Last Updated On: March 8th, 2013] [Originally Added On: March 8th, 2013]
- Being aware of low testosterone - Video [Last Updated On: March 23rd, 2013] [Originally Added On: March 23rd, 2013]
- Low Testosterone, or so I thought. - Video [Last Updated On: April 9th, 2013] [Originally Added On: April 9th, 2013]
- Haas Psychiatric Wellness - Low Testosterone - Video [Last Updated On: April 20th, 2013] [Originally Added On: April 20th, 2013]
- low testosterone linked to future ra - Video [Last Updated On: May 19th, 2013] [Originally Added On: May 19th, 2013]
- www.YourTScore.com Importance of Testing for Low Testosterone by Dr. Tammy Tucker - Video [Last Updated On: May 24th, 2013] [Originally Added On: May 24th, 2013]
- Low testosterone= Low Sex Drive - Video [Last Updated On: June 15th, 2013] [Originally Added On: June 15th, 2013]
- The Chicken or the Egg: Which Comes First, Diabetes or Low Testosterone in Men? - Video [Last Updated On: July 2nd, 2013] [Originally Added On: July 2nd, 2013]
- Dr. DiPiazza Updates Low Testosterone - Video [Last Updated On: July 8th, 2013] [Originally Added On: July 8th, 2013]
- About Low Testosterone and Treatment - Video [Last Updated On: July 16th, 2013] [Originally Added On: July 16th, 2013]
- SHC Video 12 Low Testosterone - Video [Last Updated On: July 18th, 2013] [Originally Added On: July 18th, 2013]
- Anti Aging Costa Rica - What is LowT? ( What is Low Testosterone? ) and Anti-Aging Costa Rica - Video [Last Updated On: May 4th, 2015] [Originally Added On: July 18th, 2013]
- The Doctor is In: Low testosterone - Video [Last Updated On: July 28th, 2013] [Originally Added On: July 28th, 2013]
- How Is Low T Or Low Testosterone Diagnosed? By Low T Expert Dr David Asher In Orange County CA - Video [Last Updated On: August 7th, 2013] [Originally Added On: August 7th, 2013]
- Low T Or Low Testosterone Introduction By Low T Expert Dr David Asher MD in Anaheim - Video [Last Updated On: August 7th, 2013] [Originally Added On: August 7th, 2013]
- Low T Or Low Testosterone Quiz With Dr David Asher MD in Anaheim OC Orange County - Video [Last Updated On: August 7th, 2013] [Originally Added On: August 7th, 2013]
- Low T Quiz With Low Testosterone Expert Dr David Asher MD in Anaheim CA VIDEO - Video [Last Updated On: August 10th, 2013] [Originally Added On: August 10th, 2013]
- Diet soda vs. regular; low testosterone: Healthy Living [Last Updated On: March 17th, 2025] [Originally Added On: August 14th, 2013]
- Natural Cure For Low Testosterone Helps To Boost Health And Vitality - Video [Last Updated On: August 18th, 2013] [Originally Added On: August 18th, 2013]
- Natural Herbal Remedies For Low Testosterone Help To Improve Sex Drive In Men - Video [Last Updated On: August 18th, 2013] [Originally Added On: August 18th, 2013]
- Build up your keywords low testosterone, Tyler-Longview Texas - Video [Last Updated On: September 2nd, 2013] [Originally Added On: September 2nd, 2013]
- Build up your low testosterone, Tyler-Longview Texas - Video [Last Updated On: September 2nd, 2013] [Originally Added On: September 2nd, 2013]
- Low Testosterone - Clinical Research Study - Video [Last Updated On: September 6th, 2013] [Originally Added On: September 6th, 2013]
- low estrogen vs low testosterone in men - Video [Last Updated On: September 18th, 2013] [Originally Added On: September 18th, 2013]
- Low Testosterone Treatment with Frisco Family Physician Dr. Bradley Friedman - Video [Last Updated On: September 26th, 2013] [Originally Added On: September 26th, 2013]
- Herbs for low testosterone - Video [Last Updated On: October 4th, 2013] [Originally Added On: October 4th, 2013]
- Food Items To Treat Low Testosterone Problems - Video [Last Updated On: October 5th, 2013] [Originally Added On: October 5th, 2013]
- 5 Natural Ways To Treat Low Testosterone - Video [Last Updated On: October 8th, 2013] [Originally Added On: October 8th, 2013]
- How low testosterone could be deadly - Video [Last Updated On: October 23rd, 2013] [Originally Added On: October 23rd, 2013]
- Low T Treatment | Low Testosterone Treatments | Low Testosterone ... [Last Updated On: January 26th, 2018] [Originally Added On: November 2nd, 2013]
- Is it Low T | Signs, Symptoms, and what you can do about Low T [Last Updated On: December 7th, 2017] [Originally Added On: November 3rd, 2013]
- enVoqueMD talks low testosterone, how to increase levels - Video [Last Updated On: November 3rd, 2013] [Originally Added On: November 3rd, 2013]
- Low Testosterone Causes Fatigue - Video [Last Updated On: November 5th, 2013] [Originally Added On: November 5th, 2013]
- Something Every Man Needs to Know - Video [Last Updated On: November 6th, 2013] [Originally Added On: November 6th, 2013]
- Low Testosterone - WebMD: Symptoms, Health Effects, and ... [Last Updated On: December 2nd, 2017] [Originally Added On: November 12th, 2013]
- Low Testosterone (Low T) Levels in Men, Women, Symptoms - OnHealth [Last Updated On: December 1st, 2017] [Originally Added On: November 12th, 2013]
- Low Testosterone (Low T) Symptoms, Causes, and Treatment [Last Updated On: March 26th, 2020] [Originally Added On: November 12th, 2013]
- Low T Treatment | Low Testosterone Treatments | Low ... [Last Updated On: January 13th, 2018] [Originally Added On: November 18th, 2013]
- Is it Low Testosterone? [Last Updated On: January 5th, 2018] [Originally Added On: November 21st, 2013]
- Testosterone Pills For Men [Last Updated On: March 29th, 2025] [Originally Added On: November 23rd, 2013]
- Testosterone: Indications, Side Effects, Warnings - Drugs.com [Last Updated On: December 28th, 2017] [Originally Added On: December 2nd, 2013]
- Low Testosterone (Low-T) Symptoms, Causes, Treatment - What ... [Last Updated On: December 3rd, 2017] [Originally Added On: December 3rd, 2013]
- Low Testosterone in Women - Video [Last Updated On: December 6th, 2013] [Originally Added On: December 6th, 2013]
- Quack Medicine? A Push to Sell Testosterone Gels Is Not Without Major Side Effects [Last Updated On: April 4th, 2025] [Originally Added On: December 14th, 2013]
- Gym Time - Video [Last Updated On: December 19th, 2013] [Originally Added On: December 19th, 2013]
- Low Testosterone and Your Health - WebMD Men's Health Center ... [Last Updated On: January 30th, 2018] [Originally Added On: December 21st, 2013]
- Low testosterone signs, symptoms and treatments Dr Sam Chawla by Test X180 Ignite - Video [Last Updated On: January 2nd, 2014] [Originally Added On: January 2nd, 2014]
- Low Testosterone Symptoms In Men - Video [Last Updated On: January 3rd, 2014] [Originally Added On: January 3rd, 2014]
- Hormone therapies help older adults find new life [Last Updated On: December 8th, 2017] [Originally Added On: January 8th, 2014]
- HORMONES DECREASE AS PEOPLE AGE, BUT REMEDIES HELP OLDER ADULTS FIND NEW LIFE [Last Updated On: January 10th, 2018] [Originally Added On: January 8th, 2014]
- Accumed Research Associates Featured On Katz's Corner 77 WABC Radio Show To Discuss Enlarged Prostate Treatment and ... [Last Updated On: January 7th, 2018] [Originally Added On: January 13th, 2014]
Word Count: 364