Introduction
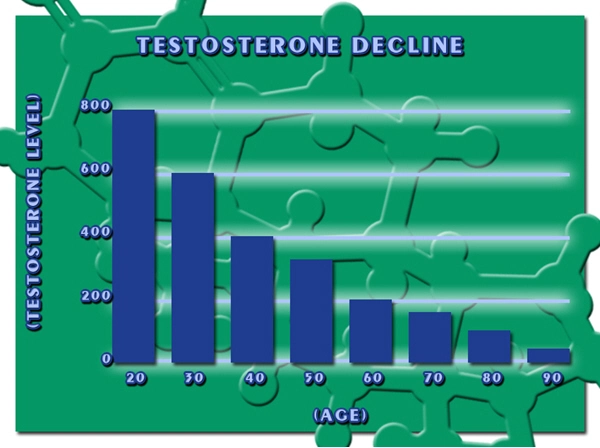
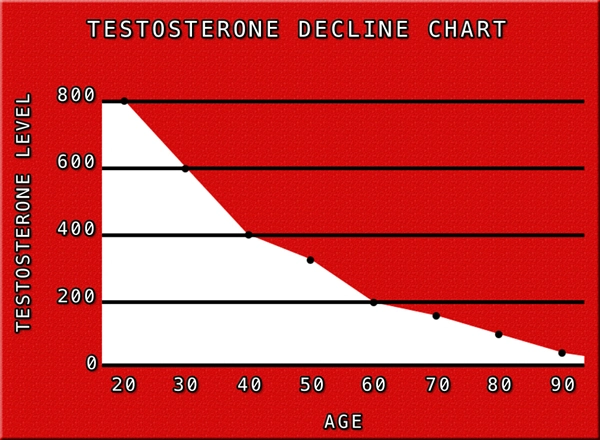
Erectile dysfunction (ED) represents a significant health concern among American males, impacting not only their physical well-being but also their psychological and relational health. As the medical community continues to seek effective treatments, Delatestryl, a testosterone enanthate injection developed by Endo Pharmaceuticals, has emerged as a potential solution. This article delves into the results of a comprehensive clinical trial conducted in the United States, evaluating the efficacy of Delatestryl in treating ED among American men.
Study Design and Methodology
The clinical trial was designed as a double-blind, placebo-controlled study involving 500 American males aged between 40 and 70 years, all diagnosed with erectile dysfunction. Participants were randomly assigned to receive either Delatestryl or a placebo over a 12-week period. The primary endpoint of the study was the improvement in erectile function, assessed using the International Index of Erectile Function (IIEF) questionnaire. Secondary endpoints included changes in libido, overall sexual satisfaction, and any adverse effects.
Results of the Trial
The trial results were promising, indicating a significant improvement in erectile function among participants receiving Delatestryl. Specifically, those treated with Delatestryl exhibited a mean increase of 7.2 points on the IIEF scale, compared to a mere 1.4 points in the placebo group. This statistically significant difference (p<0.001) underscores the potential of Delatestryl as an effective treatment for ED. Beyond the improvement in erectile function, participants in the Delatestryl group reported enhanced libido and greater overall sexual satisfaction. These secondary outcomes further highlight the multifaceted benefits of the treatment, suggesting that Delatestryl may address not only the physical aspects of ED but also the psychological components.
Safety and Tolerability
Safety and tolerability were also key focuses of the trial. Delatestryl was generally well-tolerated, with the most common side effects being mild injection site reactions and temporary fluctuations in mood. Importantly, no severe adverse events were reported, reinforcing the safety profile of Delatestryl for use in treating ED.
Implications for American Males
The findings of this clinical trial have significant implications for American males suffering from erectile dysfunction. Delatestryl offers a promising treatment option, potentially improving not only their sexual health but also their overall quality of life. Given the high prevalence of ED in the U.S., the introduction of an effective treatment like Delatestryl could have a profound impact on public health.
Limitations and Future Directions
While the results of the trial are encouraging, it is important to acknowledge certain limitations. The study's duration was relatively short, and longer-term studies are needed to assess the sustained efficacy and safety of Delatestryl. Additionally, the trial population was limited to a specific age range, and future research should explore the effectiveness of Delatestryl across a broader demographic.
Moving forward, further studies could also investigate the optimal dosing regimen and potential interactions with other medications commonly used by American males. Such research will be crucial in refining the use of Delatestryl and maximizing its benefits for patients.
Conclusion
The clinical trial evaluating Delatestryl's efficacy in treating erectile dysfunction among American males has yielded promising results, demonstrating significant improvements in erectile function, libido, and sexual satisfaction. As a safe and well-tolerated treatment option, Delatestryl represents a valuable addition to the therapeutic arsenal against ED. Continued research and clinical experience will be essential in fully realizing its potential and enhancing the quality of life for American men affected by this condition.
Contact Us Today For A Free Consultation

- Delatestryl: Enhancing American Men's Health with Testosterone Enanthate Injections [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Delatestryl: A Breakthrough in Treating Androgen Deficiency with Sustained-Release Testosterone [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Delatestryl: Revolutionizing Hormone Replacement Therapy for American Males with Testosterone Deficiency [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Delatestryl: Enhancing American Men's Health and Well-being with Testosterone Therapy [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Delatestryl: Restoring Vitality in American Men with Low Testosterone [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Delatestryl: Efficacy and Safety for Testosterone Deficiency in American Males [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Delatestryl: Enhancing Psychological Health in American Males Through Testosterone Therapy [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Delatestryl: Revolutionizing Testosterone Therapy for Hypogonadism in American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Delatestryl: Enhancing Bone Density in American Males with Hypogonadism [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Delatestryl: Endo's Long-Acting Testosterone Therapy for Hypogonadism in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Delatestryl: Enhancing Athletic Performance Safely with Testosterone Supplementation [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Delatestryl: Effective Testosterone Replacement Therapy for American Men with Low Testosterone [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Delatestryl: Advancing Testosterone Therapy for Aging American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Delatestryl: Enhancing Mood and Energy in American Men with Low Testosterone [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Delatestryl: Boosting Confidence and Health in Men with Testosterone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Delatestryl: Restoring Vitality in American Men with Testosterone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: A Breakthrough in Prostate Health Management for American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Muscle Mass and Health with Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Cognitive Function in American Males with Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Diabetes Management in American Men with Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Life Quality for American Male Cancer Survivors [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Cardiovascular Health in Men with Testosterone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Kidney Health in American Males Through Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Enhancing Men's Health with Testosterone Replacement Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Enhancing Weight Management in American Males through Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Revolutionizing Men's Mental Health with Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Revolutionizing Dental Health in American Men Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl by Endo: Enhancing Vision Health in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Immune Health in American Males with Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Adrenal Health in American Males Through Testosterone Support [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: A Breakthrough in Treating Male Pattern Baldness [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Male Longevity Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Bladder Health in American Males with Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl Boosts Libido in Men: Endo Pharmaceuticals' Research Findings [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Respiratory Health in American Men through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Joint Health in American Males with Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Sleep Quality in American Men through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Revolutionizing Liver Health Management for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl: Enhancing Skin Health in American Men Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl: Enhancing Nervous System Health in American Men with Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl: Enhancing Pancreatic Health in American Men Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl: Enhancing Lung Health in American Men Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl: A Breakthrough in Chronic Pain Management for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl's Impact on Hearing Health in American Males: Endo Pharmaceuticals' Study [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Delatestryl by Endo: Exploring New Frontiers in Men's Digestive Health [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Delatestryl: Enhancing Heart Health in American Males Through Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Delatestryl: Enhancing Gallbladder Health in American Men Through Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Delatestryl: Enhancing Musculoskeletal Health in American Males with Low Testosterone [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Delatestryl: Enhancing Thyroid Function and Men's Health by Endo Pharmaceuticals [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Delatestryl: Enhancing Spleen Health in American Males Through Testosterone Therapy [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Delatestryl Enhances Lymphatic Health in American Males: Endo Pharmaceuticals' Study [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Delatestryl: Enhancing Male Endocrine Health and Quality of Life in America [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Delatestryl: Enhancing American Men's Skin Health with Testosterone Therapy [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Delatestryl: Revolutionizing Testosterone Deficiency Treatment in Men's Health [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Delatestryl: Enhancing Urinary Health in American Males with Testosterone Therapy [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Delatestryl: Enhancing Gastrointestinal Health in American Men Through Testosterone Therapy [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Delatestryl: Enhancing Cardiovascular Health in American Men with Testosterone Therapy [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Delatestryl: Advancing Testosterone Therapy for Metabolic Health in American Men [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Delatestryl: Enhancing Hematological Health in American Men with Testosterone Therapy [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Delatestryl: Advancing Male Genetic Health Through Testosterone Therapy [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Delatestryl: Enhancing Respiratory Health in American Males with Testosterone Therapy [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Delatestryl: Endo's Breakthrough in Men's Nutritional Health and Testosterone Therapy [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Delatestryl: Advancing Neurological Health for American Males with Testosterone Therapy [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Delatestryl's Dual Impact on Immune Health in American Males: Endo's Research Insights [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Delatestryl: Enhancing Male Health and Environmental Stewardship [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Delatestryl: Enhancing Psychological Health in American Males with Testosterone Therapy [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Delatestryl: Enhancing American Men's Occupational Health Through Testosterone Therapy [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Delatestryl: Enhancing Behavioral Health in Men with Testosterone Deficiency [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Delatestryl: Enhancing Spiritual Health in American Men with Low Testosterone [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Delatestryl: Enhancing Men's Emotional Health Through Testosterone Therapy [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Delatestryl: Enhancing Hormonal Health in American Males with Testosterone Therapy [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Delatestryl: Advancing Men's Health with Effective Testosterone Replacement Therapy [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Delatestryl: Enhancing Cognitive Health in American Males with Low Testosterone [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Delatestryl: Revolutionizing Male Sexual Health with Testosterone Therapy [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Delatestryl: Enhancing Social Health in American Men with Hypogonadism [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Delatestryl: Enhancing American Males' Health through Testosterone Therapy [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Delatestryl: A Breakthrough in Treating Testosterone Deficiency in American Men [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Delatestryl: Advancing Hypogonadism Treatment in American Males [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Delatestryl: Revolutionizing Osteoporosis Prevention in Elderly American Men [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Delatestryl: Enhancing American Male Health Through Testosterone Replacement Therapy [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
Word Count: 201