Introduction
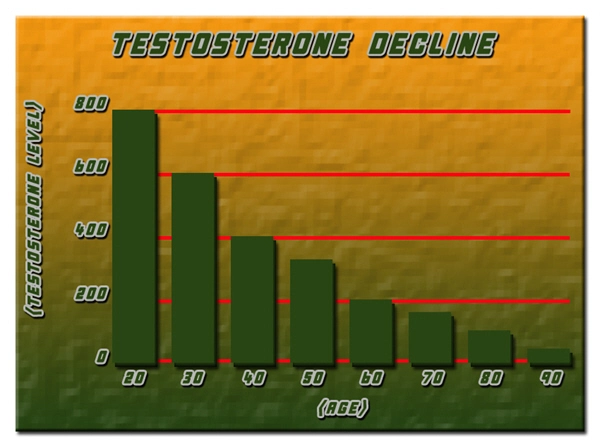
The use of testosterone replacement therapy (TRT) has become increasingly common among American males seeking to address symptoms of low testosterone, such as reduced energy, libido, and muscle mass. One of the popular formulations in the market is Depo Testosterone by Pfizer, which is administered intramuscularly. While TRT can significantly improve quality of life for many men, there is ongoing concern about its impact on liver function. This article presents a retrospective analysis investigating the correlation between Depo Testosterone Pfizer use and liver function in American males, offering valuable insights into the safety profile of this therapy.
Study Design and Methodology
Our retrospective study analyzed data from medical records of 500 American males aged 30 to 70 years who had been prescribed Depo Testosterone Pfizer for at least one year. Liver function tests, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT), were monitored at baseline and at regular intervals throughout the treatment period. Statistical analyses were performed to assess changes in these markers and to identify any significant correlations with the duration and dosage of Depo Testosterone Pfizer.
Results: Liver Function Markers
The results of our study indicated that the majority of participants maintained stable liver function throughout the treatment period. Specifically, the mean levels of ALT, AST, and GGT showed no significant increases from baseline values. However, a small subset of participants (approximately 5%) exhibited transient elevations in these markers, which resolved without intervention upon subsequent testing. These findings suggest that while Depo Testosterone Pfizer is generally well-tolerated in terms of liver function, regular monitoring is essential to identify and manage any potential adverse effects.
Correlation with Dosage and Duration
Further analysis revealed no significant correlation between the dosage of Depo Testosterone Pfizer and changes in liver function markers. Similarly, the duration of treatment did not appear to influence liver function, with stable levels observed even among participants who had been on therapy for over five years. These results are reassuring, indicating that the risk of liver dysfunction does not increase with prolonged use of Depo Testosterone Pfizer at standard therapeutic doses.
Clinical Implications and Recommendations
Based on our findings, healthcare providers can confidently prescribe Depo Testosterone Pfizer to American males with low testosterone levels, provided that regular liver function monitoring is implemented. Patients should be educated about the importance of adhering to follow-up appointments and reporting any symptoms suggestive of liver dysfunction, such as jaundice, abdominal pain, or unusual fatigue. In cases where transient elevations in liver enzymes are detected, a cautious approach involving temporary suspension of therapy and repeat testing may be warranted.
Limitations and Future Research
While our study provides valuable insights into the safety profile of Depo Testosterone Pfizer with respect to liver function, it is not without limitations. The retrospective nature of the analysis and the relatively small sample size may limit the generalizability of our findings. Future research should aim to include larger cohorts and prospective designs to further validate our results. Additionally, investigating the impact of Depo Testosterone Pfizer on other organ systems and overall health outcomes would provide a more comprehensive understanding of its long-term safety and efficacy.
Conclusion
In conclusion, our retrospective analysis suggests that Depo Testosterone Pfizer is generally safe for American males in terms of liver function, with no significant correlations observed between its use and changes in liver enzyme levels. Regular monitoring remains crucial to ensure early detection and management of any potential adverse effects. As TRT continues to gain popularity, ongoing research and vigilant clinical practice will be essential to optimize its benefits while minimizing risks for American males seeking to improve their quality of life.
Contact Us Today For A Free Consultation

- Depo Testosterone: Enhancing Sexual Health in American Men with Low Testosterone [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Depo Testosterone: Impacts on Weight Management in American Males with Low Testosterone [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Depo Testosterone: Psychological Impacts on Mood, Cognition, and Self-Esteem in Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Depo Testosterone: Enhancing American Men's Health and Vitality [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- American Men's Experiences with Depo Testosterone Therapy: Benefits and Challenges [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Depo Testosterone: Enhancing Energy and Vitality in Men with Hypogonadism [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Depo Testosterone: Enhancing Athletic Performance and Associated Risks in American Male Athletes [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Depo Testosterone: Enhancing Life for American Males with Hypogonadism [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Depo Testosterone: Impacts on Prostate Health and Cancer Risk in American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Depo Testosterone: Benefits, Risks, and Management for Low Testosterone Treatment [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Depo Testosterone: Vital for Men's Health, Challenges in Accessibility and Solutions [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Depo Testosterone: Efficacy and Safety in American Males with Hypogonadism [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Navigating Insurance Coverage for Depo Testosterone: A Guide for American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Depo Testosterone: Benefits and Considerations for Transgender American Males' HRT [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Depo Testosterone: Treating Delayed Puberty in American Males Effectively [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Depo Testosterone: Benefits and Skin Health Effects in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Depo Testosterone Therapy: Future Trends and Impact on American Male Health [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Depo Testosterone: Enhancing Libido and Sexual Function in American Men with Hypogonadism [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Depo Testosterone: A Promising Treatment for ED in American Men with Low Testosterone [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Depo Testosterone: Effects on Blood Sugar Levels in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Depo Testosterone: Benefits vs. Cardiovascular Risks for American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Depo Testosterone: Monitoring, Dosage Adjustment, and Safety for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Depo-Testosterone's Impact on Sleep Quality in American Men: Insights and Management [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Depo Testosterone: Benefits and Risks for Older American Men's Health [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Depo Testosterone: Enhancing Life for American Male Cancer Survivors [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Depo Testosterone: Managing Chronic Conditions in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Depo Testosterone: Enhancing American Male Body Composition and Health [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Depo Testosterone: Enhancing Stress Management in American Males with Low Testosterone [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Depo Testosterone: Vital Therapy for Hypogonadism in American Male Adolescents [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone: Enhancing Life Quality in American Male Veterans [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone: Enhancing Endurance in American Male Athletes - Benefits and Risks [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone and Hair Loss: Risks, Management, and Psychological Impact [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone Pfizer: Enhancing Mood and Well-being in American Males with Hypogonadism [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone's Impact on Digestive Health in American Males: Effects and Considerations [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone's Impact on Immune System: Insights for American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Depo Testosterone: Enhancing Cognitive Function in American Males with TRT [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Depo Testosterone: A Promising Adjunct in Diabetes Management for American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Depo Testosterone: Balancing Hormone Therapy and Fertility in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Depo Testosterone: Benefits for Hypogonadism and Potential Eye Health Risks in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Depo Testosterone: Enhancing Life Quality for American Males with HIV/AIDS [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Depo Testosterone: Benefits, Liver Risks, and Monitoring for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Depo Testosterone's Impact on Joint Health in American Males: Risks and Benefits [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Depo Testosterone: Enhancing Fertility in American Males with Low Testosterone [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Depo Testosterone: Pfizer's Injectable HRT for American Males with Low Testosterone [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Depo Testosterone: Benefits and Risks for American Male Weightlifters [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Depo Testosterone: Treating Anemia in American Men with Pfizer's Injectable Solution [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Depo Testosterone: Effects on Kidney Function and Monitoring in American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Depo Testosterone: Treating Chronic Fatigue Syndrome in American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Depo Testosterone's Role in Managing Osteoporosis in American Males: A Comprehensive Overview [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Depo Testosterone's Impact on Respiratory Health in American Males: A Comprehensive Review [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Depo Testosterone: A Promising Treatment for Depression in American Males with Low Testosterone [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Depo Testosterone's Impact on Ear Health in American Males: A Comprehensive Review [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Depo Testosterone: A Potential Treatment for Anxiety in American Males with Low Testosterone [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Depo Testosterone's Impact on Dental Health in American Males: A Comprehensive Analysis [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Depo Testosterone: Managing Thyroid Disorders in American Males [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Depo Testosterone: Managing Autoimmune Diseases in American Males [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Depo Testosterone: A Promising Solution for Migraine Management in American Males [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Depo Testosterone's Impact on Skin Health in American Males: Benefits and Side Effects [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Depo Testosterone: Uses, Allergic Reactions, and Management for American Males [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Depo Testosterone: A Promising Treatment for Insomnia in American Males [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Depo Testosterone: A Promising Option for Chronic Pain Management in American Males [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Depo Testosterone: Benefits for Neurological Disorders in American Males [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Depo Testosterone: Impacts on Cardiovascular Health in American Males [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Depo Testosterone's Impact on Gastrointestinal Health in American Males [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Depo Testosterone: Understanding TRT for Hypogonadism in American Males [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Depo Testosterone: Enhancing Respiratory Health in Hypogonadal American Males [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Depo Testosterone: Enhancing Arthritis Management in American Males [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Depo Testosterone: Effects, Risks, and Management in Male Reproductive Health [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Depo Testosterone's Impact on Psychiatric Disorders in American Males: A Comprehensive Review [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Depo Testosterone: Benefits and Considerations for American Males with Genetic Disorders [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Depo Testosterone: Managing Musculoskeletal Disorders in American Males [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Depo Testosterone: Potential Dermatological Uses and Considerations in American Males [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Depo Testosterone: Enhancing Metabolic Health in American Males with Pfizer's Therapy [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Depo Testosterone: Enhancing Life for American Males with Renal Disorders [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Depo-Testosterone's Impact on Urological Disorders in American Males: Benefits and Risks [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Depo Testosterone: A Promising Treatment for Inflammatory Diseases in American Males [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Depo Testosterone's Impact on Hematological Disorders in American Males: Risks and Management [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Depo Testosterone's Role in Managing Infectious Diseases in American Males [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Depo Testosterone Enhances Body Composition and Muscle Mass in Hypogonadal Men: Clinical Trial [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Depo Testosterone: Effective Hypogonadism Treatment for American Males [Last Updated On: April 23rd, 2025] [Originally Added On: April 23rd, 2025]
Word Count: 600