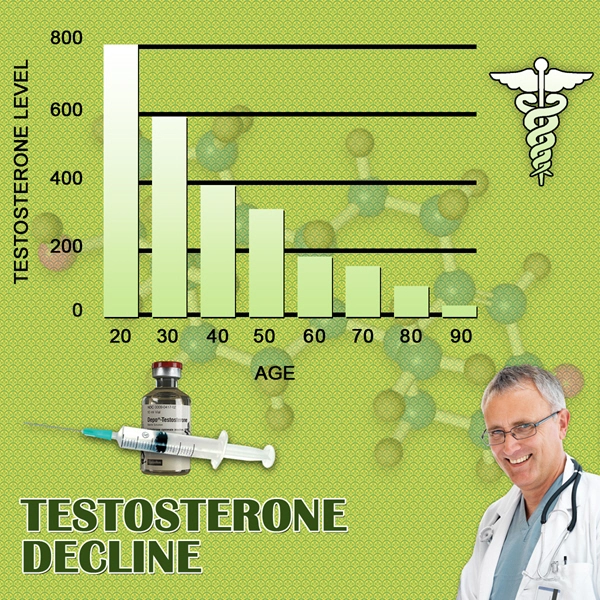
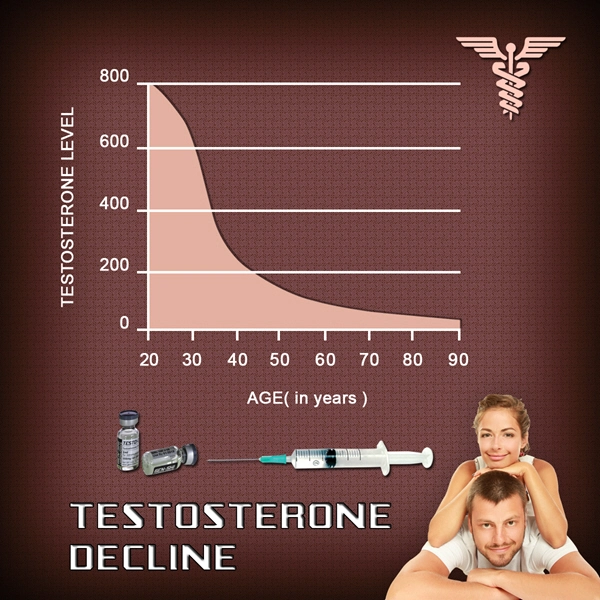
Recently, the only methods to deliver testosterone replacement therapy (TRT) were to receive an injection, swallow pills, or apply a topical gel.
Recently, the only methods to deliver testosterone replacement therapy (TRT) were to receive an injection, swallow pills, or apply a topical gel.
However, a new approach has emerged that is more convenient for some.
TRT is now available using a nasal gel that raises men’s low testosterone levels to normal, with few side effects.
Video Link: https://vimeo.com/289157199
Video Download: Click Here To Download Video
Video Stream: Click Here To Stream Video
This exciting development is based on the results of phase 3 clinical trial to be presented at the Endocrine Society’s 97th annual meeting in San Diego.
Last May, the U.S. Food and Drug Administration (FDA) approved the drug called Natesto, which is the only FDA-approved nasal testosterone replacement therapy, according to the manufacturer, Trimel Pharmaceuticals.
Under the terms of the agreement, Trimel will receive an upfront payment of $25 million and could receive additional payments upon the achievement of certain regulatory and sales milestones.
Under the terms of the agreement, Trimel will be solely responsible for the manufacture and supply of Natesto, and Endo receives exclusive commercial rights to the Natesto product in the US and Mexico.
Also, Endo will collaborate on all regulatory and clinical development activities in coordination with Trimel. And, Endo will pay a tiered supply price for the product. Endo expected the transaction to close in 2015.
"We are pleased to acquire the rights to Natesto to enhance our branded pharmaceutical portfolio further and look forward to leveraging our commercial expertise in the areas of men's health and urology to support this highly differentiated product," said Rajiv De Silva, President, and CEO of Endo.
"Natesto offers a unique intranasal delivery system which will expand options for appropriate patients seeking testosterone replacement therapy, and we are focused on getting Natesto to market as expeditiously as possible."
“The unique delivery system makes this a convenient and easy-to-use, self-administered form of testosterone to treat adult males with hypogonadism [low testosterone],” said the study’s lead investigator and a consultant to Trimel, Alan Rogol, MD, Ph.D., who is a professor emeritus at the University of Virginia,  Charlottesville.
Charlottesville.
“Also important is that intranasal testosterone minimizes the risk of unwanted secondary exposure of testosterone to women or children,” Rogol said.
Testosterone products applied to the skin have a potentially huge problem: this method of delivery risks transferring some of the drugs to others with whom the user comes into close contact.
In contrast, the new formulation sends testosterone directly into the nostril.
The product arrives in a multiple-dose pump dispenser that administers a specified amount of testosterone gel (5.5 milligrams, or mg) inside each nostril.
The phase 3 clinical trial measured the effectiveness and safety of the nasal testosterone gel in 306 men with low testosterone at 39 outpatient centers in the U.S.
Men used the treatment for 90 days in both nostrils either twice a day (228 men) or three times a day (78 men) as randomly assigned, to determine the most efficient dose.
They then stayed on the drug for another 90 or 180 days to evaluate tolerance to the medication and the effects of treatment.
After 90 days of treatment, the average testosterone concentration in the blood was in the usual range for 90 percent of men who used the nasal gel three times daily, compared with 71 percent of men using it twice a day.
(This measure was in the intent-to-treat population: all subjects who received the randomized study drug and had at least one valid post-baseline efficacy measurement.)
 As a result of the study, the manufacturer’s recommended dosing is now three times a day in each nostril, for a total daily dose of 33 mg.
As a result of the study, the manufacturer’s recommended dosing is now three times a day in each nostril, for a total daily dose of 33 mg.
Additionally, the treatment also strongly improved men’s erectile function and mood, Rogol reported.
So far, no severe side effects related to the medicine occurred in either dosing group, according to the investigators.
Rogol said there were few problems with tolerating the nasal gel, with a mere 3.7 percent of men receiving the three-times-daily dose ceasing use of the medication due to side effects.
Of 99 men who completed a survey on their experience with the drug, 84 percent felt confident they were correctly using the pump applicator within two days of beginning treatment.
“These results indicate that nasal testosterone gel is an effective and practical alternative to other available testosterone replacement therapy products,” Rogol said.
Facts you need to know about Natesto Nasal Gel
Indications and Usage
 Natesto is an androgen indicated for replacement therapy in males for conditions associated with a deficiency or absence of endogenous testosterone including:
Natesto is an androgen indicated for replacement therapy in males for conditions associated with a deficiency or absence of endogenous testosterone including:
- Primary hypogonadism (congenital or acquired)
- Hypogonadotropic hypogonadism (congenital or acquired)
Limitations of Use
Safety and efficacy of Natesto™ in males less than 18 years old have not been established
Dosage
Natesto for intranasal use is available as a metered-dose pump.
One pump actuation delivers 5.5 mg of testosterone.
The recommended dose of Natesto™ is 11 mg of testosterone (two pump actuators, one per nostril), applied intranasally three times daily for a total daily dose of 33 mg.
Contraindications
- Men with carcinoma of the breast or known or suspected prostate cancer must exercise extreme caution, and work in close collaboration with their physician to monitor any adverse developments. Specifically, this includes monitoring serum testosterone, prostate-specific antigen (PSA), hemoglobin, hematocrit, liver function tests, and lipid concentrations periodically
- Pregnant or breastfeeding women should not use Natesto, since testosterone may cause fetal harm
Warnings and Precautions
- Nasal adverse reactions: nasal signs and symptoms should be monitored. Natesto is not recommended for use in patients with chronic nasal conditions or alterations in nasal anatomy
- Monitor patients with benign prostatic hyperplasia (BPH) for worsening of signs and symptoms of BPH
- Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), have been reported in patients using testosterone products. Evaluate patients with signs or symptoms consistent with DVT or PE
- Women and children should not use Natesto
- Exogenous administration of androgens may lead to azoospermia, which is defined as “the medical condition of a man not having any measurable level of
 sperm in his semen.”
sperm in his semen.” - Edema with or without congestive heart failure (CHF) may be a complication in patients with preexisting cardiac, renal (kidney), or hepatic (liver) disease
- Sleep apnea may occur in those with risk factors
- The most common adverse reactions (incidence >/=3%) to Natesto observed in clinical trials were an increase in prostate-specific antigen (PSA), headache, rhinorrhea, epistaxis, nasal discomfort, nasopharyngitis, bronchitis, upper respiratory tract infection, sinusitis, and nasal scab.
Reference
Contact Us Today For A Free Consultation

- Adverse Effects of Testosterone Therapy in Adult Men: A Systematic Review and Meta-Analysis [Last Updated On: July 2nd, 2024] [Originally Added On: June 4th, 2010]
- Low Testosterone Levels, Foods That Increase Testosterone Levels wwwSelf-Improvement-Bible.com [Last Updated On: November 12th, 2023] [Originally Added On: May 30th, 2011]
- Low Testosterone in Men: The Next Big Thing in Medicine! - Abraham Morgentaler, MD [Last Updated On: May 7th, 2023] [Originally Added On: June 3rd, 2011]
- How To Determine Testosterone Levels By Looking At Your Ring Finger [Last Updated On: December 7th, 2017] [Originally Added On: June 30th, 2011]
- Prolab Horny Goat Weed Testosterone Booster Supplement Review [Last Updated On: November 23rd, 2023] [Originally Added On: July 19th, 2011]
- The Healthy Skeptic: Products make testosterone claims [Last Updated On: August 13th, 2024] [Originally Added On: September 11th, 2011]
- How To Naturally Increase Testosterone [Last Updated On: November 21st, 2023] [Originally Added On: September 28th, 2011]
- Testosterone Production - Video [Last Updated On: November 25th, 2024] [Originally Added On: November 20th, 2011]
- Testosterone makes us less cooperative and more egocentric, study finds [Last Updated On: January 23rd, 2018] [Originally Added On: February 1st, 2012]
- Testosterone makes us less cooperative and more egocentric [Last Updated On: January 24th, 2018] [Originally Added On: February 1st, 2012]
- Too much testosterone makes for bad decisions, tests show [Last Updated On: April 30th, 2025] [Originally Added On: February 1st, 2012]
- Today in Research: Testosterone's Negative Effects; Diet Soda Death [Last Updated On: January 2nd, 2018] [Originally Added On: February 2nd, 2012]
- Testosterone drives ego, trips cooperation [Last Updated On: December 2nd, 2017] [Originally Added On: February 4th, 2012]
- FDA approves BioSante/Teva's testosterone gel [Last Updated On: April 28th, 2025] [Originally Added On: February 15th, 2012]
- 'Manly' Fingers Make For Strong Jawline in Young Boys [Last Updated On: December 1st, 2017] [Originally Added On: February 15th, 2012]
- Teva, BioSante Win U.S. Approval for Testosterone Therapy [Last Updated On: December 10th, 2017] [Originally Added On: February 15th, 2012]
- BioSante Gains on Approval of Testosterone Gel: Chicago Mover [Last Updated On: January 8th, 2018] [Originally Added On: February 16th, 2012]
- BioSante soars following drug approval from FDA [Last Updated On: December 26th, 2017] [Originally Added On: February 16th, 2012]
- Antibodies, Not Hard Bodies: The Real Reason Women Drool Over Brad Pitt [Last Updated On: December 24th, 2017] [Originally Added On: February 21st, 2012]
- Almark Publishing Releases Book From Mark Rosenberg, M.D. Revealing Natural Discoveries Associated With Low ... [Last Updated On: May 3rd, 2025] [Originally Added On: February 28th, 2012]
- Testosterone Replacement Clinic Comes to Kansas City with Potential to Help Thousands of Men [Last Updated On: May 2nd, 2025] [Originally Added On: March 1st, 2012]
- Study examines the relative roles of testosterone and its metabolite, dihydrotestosterone in men [Last Updated On: December 2nd, 2017] [Originally Added On: March 7th, 2012]
- The Role of 5{alpha}-Reductase Inhibition in Men Receiving Testosterone Replacement Therapy [Editorial] [Last Updated On: December 21st, 2017] [Originally Added On: March 7th, 2012]
- Effect of Testosterone Supplementation With and Without a Dual 5{alpha}-Reductase Inhibitor on Fat-Free Mass in Men ... [Last Updated On: January 3rd, 2018] [Originally Added On: March 7th, 2012]
- Why We Like Men Who Can Keep Their Cool [Last Updated On: December 30th, 2017] [Originally Added On: March 7th, 2012]
- Testosterone And Heart Health [Last Updated On: May 1st, 2025] [Originally Added On: March 10th, 2012]
- Your Life on Testosterone: Overly Sure of Yourself, Unwilling to Listen [Last Updated On: November 25th, 2018] [Originally Added On: March 15th, 2012]
- Mayo Clinic-TGen study role testosterone may play in triple negative breast cancer [Last Updated On: December 8th, 2017] [Originally Added On: March 23rd, 2012]
- A dose of testosterone might not cure what ails you [Last Updated On: January 23rd, 2018] [Originally Added On: March 25th, 2012]
- Green tea could aid athletes hide testosterone doping [Last Updated On: December 16th, 2017] [Originally Added On: March 25th, 2012]
- TGen Study Role Testosterone May Play in Triple Negative Breast Cancer [Last Updated On: December 6th, 2017] [Originally Added On: March 26th, 2012]
- Testosterone low, but responsive to competition, in Amazonian tribe [Last Updated On: January 23rd, 2018] [Originally Added On: March 28th, 2012]
- Competition-linked bursts of testosterone are fundamental aspect of human biology, study of Amazonian tribe suggests [Last Updated On: December 25th, 2017] [Originally Added On: March 28th, 2012]
- Playing football boosts testosterone levels by 30 percent! [Last Updated On: February 4th, 2024] [Originally Added On: March 28th, 2012]
- Testosterone low, but responsive to competition, in Amazonian tribe -- with slideshow [Last Updated On: December 9th, 2017] [Originally Added On: March 28th, 2012]
- The benefits of testosterone pellet therapy [Last Updated On: January 24th, 2018] [Originally Added On: March 29th, 2012]
- Low testosterone levels cause health woes [Last Updated On: November 25th, 2018] [Originally Added On: March 30th, 2012]
- Heart Failure Patients Getting Relief from Testosterone Supplements [Last Updated On: May 5th, 2025] [Originally Added On: April 21st, 2012]
- Study Finds Fatherhood Suppresses Testosterone [Last Updated On: May 4th, 2025] [Originally Added On: May 3rd, 2012]
- Low testosterone levels could raise diabetes risk for men [Last Updated On: January 26th, 2018] [Originally Added On: May 5th, 2012]
- Why low testosterone may increase your risk of diabetes [Last Updated On: November 25th, 2024] [Originally Added On: May 5th, 2012]
- Diabetes link to low testosterone [Last Updated On: November 25th, 2024] [Originally Added On: May 5th, 2012]
- Testosterone Linked to Weight Loss in Obese Men [Last Updated On: January 2nd, 2018] [Originally Added On: May 11th, 2012]
- Testosterone may help weight loss [Last Updated On: November 25th, 2024] [Originally Added On: May 11th, 2012]
- Testosterone-fuelled infantile males might be a product of Mom's behaviour [Last Updated On: December 25th, 2017] [Originally Added On: May 11th, 2012]
- Testosterone-fueled infantile males might be a product of Mom's behavior [Last Updated On: January 6th, 2018] [Originally Added On: May 11th, 2012]
- Testosterone supplements may help obese men lose weight [Last Updated On: January 5th, 2018] [Originally Added On: May 11th, 2012]
- Testosterone supplements 'can help men lose their middle-aged spread' [Last Updated On: November 25th, 2024] [Originally Added On: May 12th, 2012]
- Some doctors question safety of testosterone replacement therapy [Last Updated On: January 20th, 2018] [Originally Added On: May 15th, 2012]
- Health Canada Approves New Testosterone Topical Solution for Men [Last Updated On: May 15th, 2025] [Originally Added On: May 15th, 2012]
- Environment trumps genes in testosterone levels, study finds [Last Updated On: May 8th, 2025] [Originally Added On: May 15th, 2012]
- Global Testosterone Replacement Therapy (TRT) Industry [Last Updated On: May 7th, 2025] [Originally Added On: May 21st, 2012]
- Testosterone Fuels Boom, Swindler Sows Panic: Top Business Books [Last Updated On: January 13th, 2018] [Originally Added On: June 2nd, 2012]
- Increase in testosterone drug use [Last Updated On: April 12th, 2018] [Originally Added On: June 4th, 2012]
- Testosterone Promotes Agression Automatically [Last Updated On: January 29th, 2018] [Originally Added On: June 9th, 2012]
- Testosterone shown to help sexually frustrated women [Last Updated On: January 27th, 2018] [Originally Added On: June 9th, 2012]
- Research and Markets: Testosterone Replacement Therapy (TRT) - Global Strategic Business Report [Last Updated On: December 23rd, 2017] [Originally Added On: June 12th, 2012]
- Proposed testosterone testing of some female olympians challenged by Stanford scientists [Last Updated On: January 30th, 2018] [Originally Added On: June 14th, 2012]
- Testosterone Makes Bosses Into Jerks, Says Paul Zak [Last Updated On: January 8th, 2018] [Originally Added On: June 14th, 2012]
- Testosterone Therapy: A Misguided Approach to Erectile Dysfunction (ED) [Last Updated On: May 10th, 2025] [Originally Added On: June 20th, 2012]
- New drugs, new ways to target androgens in prostate cancer therapy [Last Updated On: January 8th, 2018] [Originally Added On: June 20th, 2012]
- Long-term testosterone treatment for men results in reduced weight and waist size [Last Updated On: January 19th, 2018] [Originally Added On: June 23rd, 2012]
- Declining testosterone levels in men not part of normal aging, study finds [Last Updated On: December 27th, 2017] [Originally Added On: June 23rd, 2012]
- Low testosterone not normal part of aging [Last Updated On: December 22nd, 2017] [Originally Added On: June 25th, 2012]
- Testosterone Does Not Necessarily Wane With Age [Last Updated On: December 6th, 2017] [Originally Added On: June 25th, 2012]
- Overweight men can boost low testosterone levels by losing weight [Last Updated On: December 10th, 2017] [Originally Added On: June 25th, 2012]
- Testosterone-replacement therapy improves symptoms of metabolic syndrome [Last Updated On: January 14th, 2018] [Originally Added On: June 26th, 2012]
- Testosterone therapy takes off pounds [Last Updated On: December 11th, 2017] [Originally Added On: June 26th, 2012]
- Weight loss may boost men's testosterone [Last Updated On: May 9th, 2025] [Originally Added On: June 27th, 2012]
- Low Testosterone? Study finds age may not be to blame [Last Updated On: May 12th, 2025] [Originally Added On: July 1st, 2012]
- Do you have low testosterone? [Last Updated On: December 15th, 2017] [Originally Added On: July 8th, 2012]
- Wall Streeters Buying Testosterone for an Edge [Last Updated On: May 11th, 2025] [Originally Added On: July 12th, 2012]
- Beefy Wall Street Traders rub on testosterone [Last Updated On: February 20th, 2024] [Originally Added On: July 12th, 2012]
- Tale of two runners exposes flawed Olympic thinking [Last Updated On: December 23rd, 2024] [Originally Added On: July 19th, 2012]
- Genetic markers for testosterone and estrogen level regulation identified [Last Updated On: January 6th, 2018] [Originally Added On: July 20th, 2012]
- BUSM researchers identify genetic markers for testosterone, estrogen level regulation [Last Updated On: December 18th, 2017] [Originally Added On: July 20th, 2012]
- DRS. OZ AND ROIZEN: How to reap the benefits of normal testosterone levels [Last Updated On: December 23rd, 2024] [Originally Added On: July 21st, 2012]
- How Testosterone Drives History [Last Updated On: December 24th, 2024] [Originally Added On: July 22nd, 2012]
- Testosterone replacement is "fountain of youth" for men [Last Updated On: January 3rd, 2018] [Originally Added On: July 27th, 2012]
- Pill for low testosterone in men heads for phase II clinical trials [Last Updated On: December 31st, 2017] [Originally Added On: August 2nd, 2012]
Word Count: 1054