Introduction
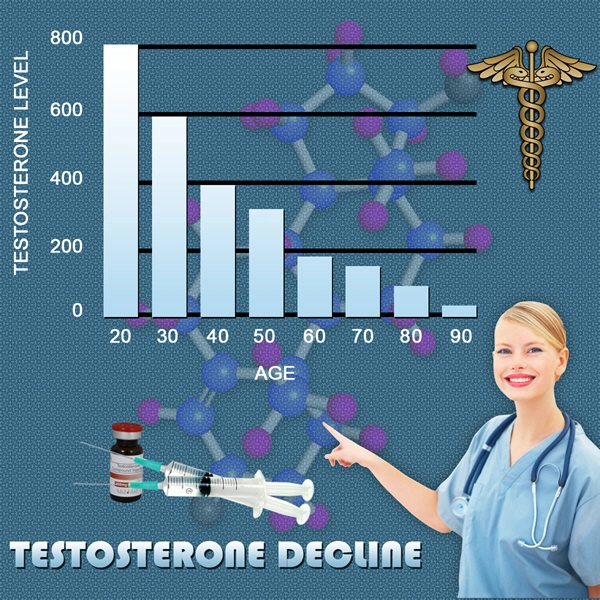
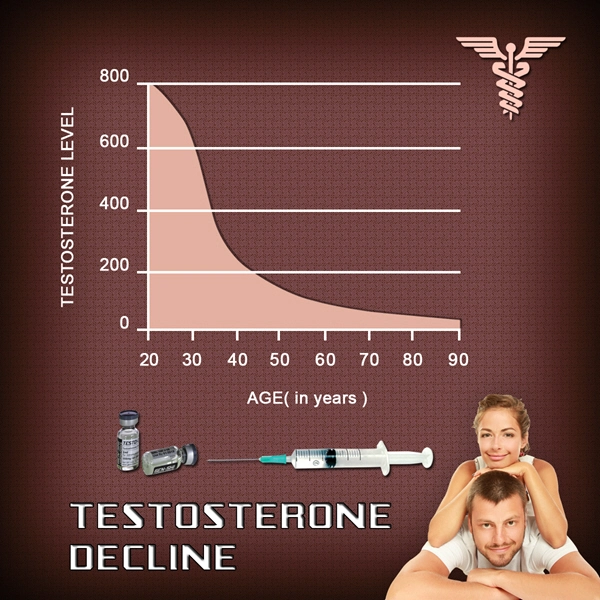
Testosterone replacement therapy (TRT) has become increasingly popular among American males seeking to address symptoms associated with hypogonadism, such as decreased libido, fatigue, and muscle loss. Among the various forms of TRT, Vogelxo testosterone gel has gained attention due to its ease of use and purported efficacy. However, the long-term effects of this treatment on liver function and hepatotoxicity remain a subject of concern. This article presents a comprehensive four-year prospective study aimed at evaluating the impact of Vogelxo testosterone gel on liver health in American males.
Study Design and Methodology
The study was conducted over a period of four years, involving 500 American males aged between 30 and 65 years who were prescribed Vogelxo testosterone gel for hypogonadism. Participants were monitored at regular intervals to assess changes in liver function through a series of biochemical tests, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT). Additionally, liver ultrasound examinations were performed annually to detect any structural abnormalities.
Results on Liver Function
Over the course of the study, the majority of participants exhibited stable liver function parameters. At the baseline, the average ALT level was 25 U/L, AST was 22 U/L, and GGT was 30 U/L. After four years of Vogelxo testosterone gel use, these values remained largely unchanged, with average ALT at 26 U/L, AST at 23 U/L, and GGT at 32 U/L. These findings suggest that Vogelxo testosterone gel does not significantly alter liver function in most American males.
Incidence of Hepatotoxicity
Despite the overall stability in liver function, a small subset of participants (approximately 3%) developed elevated liver enzymes indicative of hepatotoxicity. These individuals were closely monitored, and in most cases, the elevation was transient and resolved without intervention. However, in 0.5% of the study population, persistent elevation of liver enzymes necessitated discontinuation of Vogelxo testosterone gel. Subsequent liver biopsies in these cases revealed mild hepatic steatosis, suggesting a potential link between prolonged use of the gel and liver fat accumulation.
Liver Ultrasound Findings
Annual liver ultrasound examinations revealed no significant structural changes in the majority of participants. However, in the subset of individuals who developed hepatotoxicity, mild hepatomegaly was observed in 1% of cases. This finding underscores the importance of regular monitoring in patients undergoing TRT with Vogelxo testosterone gel.
Discussion
The results of this four-year prospective study indicate that Vogelxo testosterone gel is generally well-tolerated by American males in terms of liver function. The majority of participants maintained stable liver enzyme levels throughout the study period, suggesting that the gel does not pose a significant risk of hepatotoxicity for most users. However, the small percentage of individuals who developed elevated liver enzymes highlights the need for vigilant monitoring, particularly in those with pre-existing liver conditions or risk factors for hepatic disease.
Clinical Implications and Recommendations
Healthcare providers should consider the findings of this study when prescribing Vogelxo testosterone gel to American males. While the risk of hepatotoxicity appears low, regular monitoring of liver function is essential to identify and manage any adverse effects promptly. Patients should be educated about the potential risks and encouraged to report any symptoms suggestive of liver dysfunction, such as jaundice, abdominal pain, or unexplained fatigue.
Conclusion
In conclusion, Vogelxo testosterone gel appears to be a safe option for testosterone replacement therapy in American males, with minimal impact on liver function in the majority of users. However, a small subset of individuals may be at risk of developing hepatotoxicity, necessitating regular monitoring and prompt intervention when necessary. Further research is warranted to better understand the factors contributing to hepatotoxicity in susceptible individuals and to optimize the safety of TRT with Vogelxo testosterone gel.
Contact Us Today For A Free Consultation

- Vogelxo Testosterone Gel: Benefits, Risks, and Long-Term Health Effects in American Men [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Vogelxo Testosterone Gel: Enhancing Health and Vitality in American Men [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Vogelxo: Effective Testosterone Gel for American Men's Low T Symptoms [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Vogelxo Testosterone Gel: Dermatological Effects and Management for American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Vogelxo Pharmacokinetics in American Males: Absorption, Distribution, Metabolism, Excretion [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Vogelxo's Effects on Cardiovascular Health: Risks and Benefits for American Men [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Vogelxo Gel: Restoring Vitality in American Men with Declining Testosterone [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Vogelxo Testosterone Gel: Enhancing Physical Performance in American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Vogelxo: Enhancing Male Vitality with Testosterone Gel Therapy [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Vogelxo and Sleep: Navigating Testosterone Therapy's Impact on American Men's Sleep Patterns [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Vogelxo: Enhancing Cognitive Function in American Males with Testosterone Therapy [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Vogelxo Testosterone Gel: Enhancing Emotional Well-being in American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Vogelxo Testosterone Gel: Dispelling Myths and Enhancing Understanding for American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Vogelxo: Enhancing Life for Men with Low Testosterone - Insurance and Access Guide [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Vogelxo Testosterone Gel: Tailored Solution for American Men's Hormone Therapy [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Vogelxo: Enhancing Diabetes Management in American Men with Testosterone Therapy [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Vogelxo: Enhancing Weight Management in American Males with Hypogonadism [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Vogelxo: Managing Low Testosterone in American Men - Application, Outcomes, and Safety [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Vogelxo Testosterone Gel: Enhancing American Male Health and Wellness [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Vogelxo: Enhancing Respiratory Health in American Men with Testosterone Gel [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Vogelxo: Maximizing Benefits for American Men with Low Testosterone [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Vogelxo Testosterone Gel: Enhancing Life for American Men with Hypogonadism [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Vogelxo Testosterone Gel: Benefits, Liver Risks, and Management for American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Vogelxo Testosterone Gel: Benefits, Fertility Risks, and Reproductive Health Considerations [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Vogelxo Testosterone Gel: Impacts on Hearing and Ear Health in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Vogelxo Testosterone Gel: Efficacy and Safety in American Males with Hypogonadism [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Vogelxo Testosterone Gel: Enhancing Skin Health and Rejuvenation in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Vogelxo: Enhancing Life for American Men with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Vogelxo Testosterone Gel: Impacts on Skin Health and Safety for American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Vogelxo Testosterone Gel: Impacts on Nervous System and Safety for American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Vogelxo Testosterone Gel: Impacts on Immune System in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Vogelxo's Impact on Prostate Health in American Men: Benefits and Risks [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Vogelxo Testosterone Gel: Impacts on Digestive Health in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Vogelxo: Testosterone Gel's Impact on Vision and Eye Health in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Vogelxo: Testosterone Gel for Men - Benefits, Risks, and Usage Guidelines [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Vogelxo Testosterone Gel: Effects on Blood Pressure and Heart Rate in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Vogelxo: Enhancing American Men's Vitality with Testosterone Gel [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Vogelxo: Testosterone Gel's Impact on Dental Health in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Vogelxo Testosterone Gel: Enhancing Male Health and Vitality [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Vogelxo Testosterone Gel: Enhancing Muscle Recovery and Fitness in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Vogelxo: Testosterone Gel's Impact on Hair Loss in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Vogelxo: Enhancing Joint Health in American Men Through Testosterone Gel Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Vogelxo: Enhancing Hip and Knee Health in American Men with Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Vogelxo: Enhancing Mental Health in American Men with Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Vogelxo: Enhancing Neck and Shoulder Health in American Men with Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Vogelxo: Testosterone Gel's Impact on Kidney Function in American Men with Hypogonadism [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Vogelxo: Testosterone Gel's Role in Pain Management for American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Vogelxo: Enhancing Foot and Ankle Health in Men with Low Testosterone [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Vogelxo: Enhancing American Men's Back Health Through Testosterone Therapy [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Vogelxo: Enhancing Elbow and Arm Health in American Men with Low Testosterone [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Vogelxo Testosterone Gel: Enhancing Nail Health in American Men [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Vogelxo Gel: Enhancing Hand and Wrist Health in American Men with Hypogonadism [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Vogelxo: Enhancing Chest and Abdominal Health in American Men with Testosterone Therapy [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Vogelxo: Enhancing Leg and Thigh Health in American Men with Hypogonadism [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Vogelxo's Dual Impact on American Men's Scalp Health: Benefits and Risks [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Vogelxo Testosterone Gel: Impacts and Oral Health Management for American Men [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Vogelxo: Exploring Testosterone Gel's Impact on Sinus Health in American Men [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Vogelxo: Testosterone Gel's Impact on Throat and Voice Health in Men [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Vogelxo's Impact on Lung Health in American Men: Benefits and Risks [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Vogelxo: Enhancing Facial Health in American Men Through Testosterone Gel [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Vogelxo Testosterone Gel: Impacts on Male Reproductive Health and Fertility [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Vogelxo Testosterone Gel: Enhancing Blood Health in American Men with Hypogonadism [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Vogelxo Testosterone Gel: Effects on Skin, Hair, and Nails in American Men [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Vogelxo: Enhancing Musculoskeletal Health in American Men with Hypogonadism [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Vogelxo: Enhancing Heart Health in American Men with Low Testosterone [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Vogelxo: Enhancing Urinary Health in American Men with Hypogonadism [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Vogelxo Testosterone Gel: Impacts on Lymphatic System and Health Management for American Men [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Vogelxo Testosterone Gel: Effects on Digestive System and Management Strategies for American Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Vogelxo Testosterone Gel: Enhancing Muscle, Bone, and Joint Health in American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Vogelxo: Enhancing Nervous System Health in American Men with Testosterone Gel [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Vogelxo: Testosterone Gel's Effects on Respiratory Health in Men [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Vogelxo: Enhancing Nervous System Health in American Men with Testosterone Gel [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Vogelxo: Enhancing Urinary Health in American Men with Low Testosterone [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Vogelxo Testosterone Gel: Impact on Cardiovascular Health in American Men [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Vogelxo Testosterone Gel: Benefits, Risks, and Management for American Men [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Vogelxo: Enhancing Men's Integumentary Health and Vitality with Testosterone Gel [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Vogelxo Testosterone Gel: Enhancing Male Reproductive Health and Treating Hypogonadism [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Vogelxo Testosterone Gel: Efficacy, Benefits, and Considerations for American Men [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Vogelxo Testosterone Gel: Enhancing Systemic Health in American Men with Hypogonadism [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Vogelxo Testosterone Gel: A Convenient Solution for Low T in American Males [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
Word Count: 599