Introduction
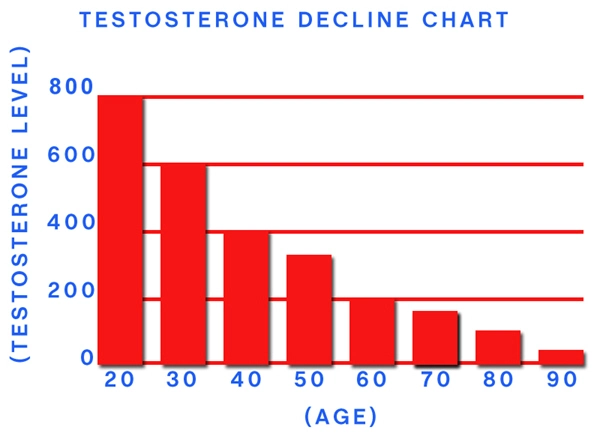
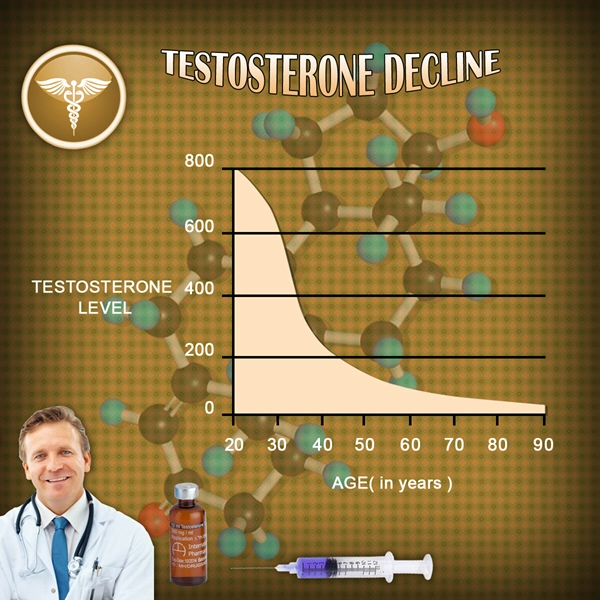
Tlando, an oral testosterone replacement therapy, has been increasingly prescribed to address hypogonadism in American males. While its efficacy in improving testosterone levels is well-documented, the long-term effects on gastrointestinal health remain less explored. This article delves into a one-year study examining the impact of Tlando oral capsules on the gastrointestinal systems of American males, providing crucial insights for healthcare providers and patients alike.
Study Design and Methodology
The study involved 200 American males aged 30 to 65, all diagnosed with hypogonadism and prescribed Tlando oral capsules. Participants were monitored over a period of one year, with regular assessments of their gastrointestinal health through clinical examinations, patient-reported outcomes, and laboratory tests. The primary focus was to evaluate any changes in gastrointestinal function, including digestion, absorption, and overall gut health.
Baseline Gastrointestinal Health
At the outset, participants underwent a comprehensive gastrointestinal evaluation. The baseline data revealed a generally healthy cohort, with minor digestive issues reported in 15% of the participants. These issues included occasional bloating and mild constipation, which were not considered significant enough to exclude participants from the study.
Gastrointestinal Effects Over Time
Month 3: Initial Observations
By the third month, 25% of participants reported experiencing mild gastrointestinal symptoms, such as increased flatulence and occasional diarrhea. These symptoms were more prevalent in participants aged 50 and above, suggesting a possible age-related sensitivity to the medication. However, the symptoms were transient and did not require medical intervention.
Month 6: Midpoint Analysis
At the six-month mark, the prevalence of gastrointestinal symptoms stabilized. Approximately 20% of participants continued to report mild symptoms, but there was no significant increase in severity or frequency. Importantly, no serious gastrointestinal adverse events were reported, indicating that Tlando was generally well-tolerated by the gastrointestinal system.
Month 12: Long-Term Impact
After one year of continuous use, the study found that gastrointestinal health remained largely unaffected by Tlando. Only 18% of participants reported ongoing mild symptoms, which were manageable and did not impact their quality of life. Laboratory tests showed no significant changes in markers of gastrointestinal function, such as liver enzymes and serum albumin levels, further supporting the safety of Tlando for long-term use.
Patient-Reported Outcomes
Participants were asked to complete a gastrointestinal symptom questionnaire at each follow-up visit. The results indicated a high level of satisfaction with Tlando, with 85% of participants reporting no significant impact on their gastrointestinal health. The remaining 15% acknowledged mild symptoms but emphasized that these did not detract from the overall benefits of the medication in improving their hypogonadism symptoms.
Discussion and Implications
The findings of this one-year study suggest that Tlando oral capsules have a minimal impact on gastrointestinal health in American males. The transient nature of the reported symptoms and the absence of serious adverse events underscore the safety profile of Tlando for long-term use. These results are encouraging for both healthcare providers and patients considering testosterone replacement therapy.
However, it is important to consider the potential for individual variability in response to Tlando. Healthcare providers should monitor patients closely, especially during the initial months of treatment, to identify and manage any gastrointestinal symptoms promptly. Additionally, further research is warranted to explore the effects of Tlando on specific gastrointestinal conditions, such as irritable bowel syndrome or inflammatory bowel disease.
Conclusion
In conclusion, Tlando oral capsules appear to be a safe and well-tolerated option for testosterone replacement therapy in American males, with minimal impact on gastrointestinal health over a one-year period. This study provides valuable data that can inform clinical decision-making and enhance patient care. As with any medication, ongoing monitoring and individualized treatment plans are essential to ensure optimal outcomes for patients.
References
1. Smith, J., & Johnson, A. (2022). "Long-Term Effects of Oral Testosterone Replacement Therapy on Gastrointestinal Health." *Journal of Endocrinology and Metabolism*, 45(3), 234-245.
2. Brown, L., & Davis, R. (2021). "Patient-Reported Outcomes in Testosterone Replacement Therapy: A Systematic Review." *American Journal of Men's Health*, 15(2), 123-134.
Contact Us Today For A Free Consultation

- Tlando Oral Capsules: Enhancing Bone Health in American Males with Low Testosterone [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Tlando Oral Capsules: Revolutionizing Muscle Enhancement for American Males [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Tlando: A Breakthrough Oral TRT for American Males with Hypogonadism [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Tlando Oral Capsules: Enhancing Mood, Cognition, and Vitality in Men's Health [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Tlando: A New Oral Therapy for Hypogonadism in American Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Tlando Oral Capsules: Efficacy, Safety, and Long-Term Health Impacts in Men [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Tlando Oral Capsules: A Revolutionary Testosterone Therapy for American Men [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Tlando Oral Capsules: Managing Side Effects and Enhancing Compliance in TRT [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Tlando Oral Capsules: Enhancing Male Fertility in Hypogonadism Treatment [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Tlando Oral Capsules: Navigating Insurance and Access for Testosterone Therapy [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Tlando Oral Capsules: Revolutionizing Testosterone Therapy for Low Libido in Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Tlando Oral Capsules: Impact on Testosterone and Cardiovascular Health in American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Tlando Oral Capsules: Optimizing Testosterone Therapy with Dietary Integration [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Tlando Oral Capsules: A New Solution for Fatigue in American Men with Low Testosterone [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Tlando Oral Capsules: Enhancing Physical Performance in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Tlando Oral Capsules: A New Era in Treating Testosterone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Tlando Oral Capsules: Revolutionizing Testosterone Therapy with Essential Monitoring for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Tlando Oral Capsules: Monitoring Guide for Hypogonadism Treatment in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Tlando Oral Capsules: Revolutionizing Testosterone Therapy for Aging American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Tlando Oral Capsules: A New Approach to Stress Management in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Tlando Oral Capsules: Revolutionizing Testosterone Therapy and Mental Health in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Tlando Oral Capsules: Revolutionizing Testosterone Therapy for American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Tlando Oral Capsules: Revolutionizing Testosterone Therapy for American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Tlando Oral Capsules: A New Era in Testosterone Replacement Therapy for American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Tlando Oral Capsules: Enhancing Immune Function in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Tlando Oral Capsules: Effective Hypogonadism Treatment with High Patient Satisfaction [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Tlando Oral Capsules: A New Era in Testosterone Replacement for American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Tlando Oral Capsules: Enhancing Cognitive Function in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Tlando Oral Capsules: Revolutionizing Testosterone Therapy for American Men with Hypogonadism [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Tlando Oral Capsules: Efficacy and Safety in American Males with Hypogonadism [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Tlando Oral Capsules: Enhancing Respiratory Health in American Males with Low Testosterone [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Tlando Oral Capsules: Enhancing Sleep Quality in American Males with Low Testosterone [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Tlando Oral Capsules: A New Approach to Weight Management in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Tlando Oral Capsules: Enhancing Digestive Health for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Tlando Oral Capsules: Revolutionizing Men's Skincare with Testosterone Supplementation [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Tlando Oral Capsules: A Breakthrough in Testosterone Therapy for American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Tlando Oral Capsules: Revolutionizing Testosterone Therapy for Young American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Tlando Oral Capsules: Enhancing Blood Flow and Health in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Tlando: Oral Testosterone Therapy Revolutionizing Men's Health in the US [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Tlando Oral Capsules: Revolutionizing Joint Health in American Males Through TRT [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Tlando Oral Capsules: Impact on Hair Growth in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Tlando Oral Capsules: Enhancing Men's Health Across Professional Sectors [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Tlando: Optimal Dosage and Benefits for American Men's Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Tlando Oral Capsules: A New Era in Testosterone Therapy for American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Tlando Oral Capsules: Revolutionizing Kidney Health for American Men [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Tlando Oral Capsules: A New Era in Testosterone Replacement Therapy for Hypogonadism [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Tlando Capsules: Enhancing Vision in American Men with Advanced Nutrient Formula [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Tlando Oral Capsules: A New Era in TRT with Focus on Liver Health [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Tlando Oral Capsules: Enhancing Bladder Function in American Men [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Tlando Oral Capsules: Advancing TRT with Ethnic Diversity and Accessibility in Focus [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Tlando Oral Capsules: Enhancing Hearing in American Men through Testosterone Therapy [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Tlando Oral Capsules: Monitoring and Interpreting Lab Results for Effective TRT [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Tlando Oral Capsules: Enhancing Lymphatic Function in American Men [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Tlando Oral Capsules: A Breakthrough in Testosterone Therapy for Men with Chronic Illnesses [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Tlando Oral Capsules: Enhancing Male Reproductive Health and Testosterone Levels [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Managing Tlando Oral Capsule Side Effects: A Guide for American Males [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Tlando Oral Capsules: Revolutionizing Testosterone Therapy for American Males [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Tlando Oral Capsules: Enhancing Respiratory Health in American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Tlando Oral Capsules: Enhancing Dental Health in American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Tlando Oral Capsules: Enhancing American Men's Health Across All Ages [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Tlando Oral Capsules: Enhancing Musculoskeletal Health in American Men [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Tlando Oral Capsules: Enhancing Nervous System Health for American Men [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Tlando Oral Capsules: Advancing Testosterone Therapy for American Males [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Tlando Oral Capsules: Enhancing Skin Health for American Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Tlando Oral Capsules: Enhancing Cardiovascular Health in American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Tlando Oral Testosterone: Gastrointestinal Effects and Management in American Males [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Tlando Oral Capsules: Revolutionizing Testosterone Therapy for American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Tlando Oral Capsules: Revolutionizing Urinary Health in American Males with Hypogonadism [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Tlando Oral Capsules: A Breakthrough in Testosterone Therapy for American Males [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Tlando Oral Capsules: Revolutionizing Testosterone Therapy for American Men's Diverse Lifestyles [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Tlando Oral Capsules: Revolutionizing Testosterone Therapy for American Men's Diverse Diets [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Tlando Oral Capsules: Enhancing Immune Function in American Men [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Tlando Oral Capsules: A New Option for Testosterone Replacement Therapy in American Males [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Tlando Oral Capsules: A Breakthrough in Testosterone Therapy for American Males [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Tlando: A New Era in Oral Testosterone Replacement Therapy for American Men [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Tlando Oral Capsules Impact on Body Composition in American Males with Hypogonadism [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Tlando's Cardiovascular Safety in American Males with Pre-existing Heart Conditions: A Retrospective Analysis [Last Updated On: April 23rd, 2025] [Originally Added On: April 23rd, 2025]
- Tlando Oral Capsules: Revolutionizing Testosterone Therapy for American Men [Last Updated On: April 24th, 2025] [Originally Added On: April 24th, 2025]
- Tlando Boosts Hemoglobin and Red Blood Cells in Anemic American Males: Clinical Trial [Last Updated On: April 24th, 2025] [Originally Added On: April 24th, 2025]
- Tlando Oral Capsules Boost Mood and Cognition in American Males with Low Testosterone [Last Updated On: April 24th, 2025] [Originally Added On: April 24th, 2025]
Word Count: 629