Reading Time: < 1 minute Lansing is the capital of the U.S. state of Michigan. It is mostly in Ingham County, although portions of the city extend west into Eaton County and north into Clinton County. The 2010 Census placed the city's population at 114,297, making it the fifth largest city in Michigan. The population of its Metropolitan Statistical Area (MSA) was 464,036, while the even larger Combined Statistical Area (CSA) population, which includes Shiawassee County, was 534,684. It was named the new state capital of Michigan in 1847, ten years after Michigan became a state. The Lansing metropolitan area, colloquially referred to as "Mid-Michigan", … Read more
- Andropause (183)
- Blog Testosterone (3)
- Diets (31)
- Drugs (9)
- Erectile Dysfunction (1)
- Fertility (7)
- HCG Injections (4)
- Hormone News (41)
- Hormone Therapy Review (54)
- How to Inject Hormones (2)
- HRT Fitness And Exercise (5)
- Human Growth Hormone (19)
- Human Growth Hormone Clinic (2)
- Hypogonadism (117)
- Hypopituitarism (2)
- Is it Low T (16)
- LabCorp Blood Testing Centers (1,809)
- Low Testosterone (242)
- Male Health (2)
- Male Sexual (3)
- Men's Health (15)
- Online Pharmacy (1)
- Quest Blood Testing Centers (1,325)
- Testosterone And Cancer (2)
- Testosterone Clinics (408)
- Testosterone Cream (37)
- Testosterone Cypionate (66)
- Testosterone Gel (96)
- Testosterone Information (1,278)
- Testosterone Injections (54)
- Testosterone News (34)
- Testosterone Replacement for Men (6)
- Testosterone Replacement Therapy (149)
- Testosterone Therapy (12)
- Omnitrope: How to Mix & Inject Omnitrope HGH Injections?
- Easily Increase Testosterone Levels and Improve Your Health Naturally: Just Follow These Tips!
- Norditropin Side Effects
- Choosing the Right Growth Hormone Therapy: A Comparison of Options
- Low T’ treatment appears to be safe for men with heart Disease.
Free Testosterone Consultation
Our Staff
* U.S. Citizens 30 Years of Age of Older Only*
Testosterone Consultants
Testosterone Products
HRT Health Categories
Testosterone Fit And Healthy
Recent Posts
Therapy Benefits
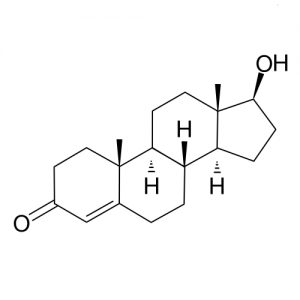
Testosterone Molecules For Health Solutions
Five Surprising Testosterone Benefits:
HGH Specialists In Testosterone Therapy
Call Us Today
Medical Forms
Testosterone Therapy
Medical Excellence
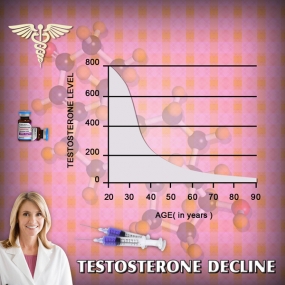
Testosterone Decline